Hydrothermally modified PVA/ZnS-NCQD nanocoating for stainless steel corrosion protection in saline water
Mohammad BinSabta,
Ahmed Abdel Nazeerab,
Metwally Madkoura and
Fakhreia Al-Sagheer*a
aChemistry Department, Faculty of Science, Kuwait University, P. O. Box 5969, Safat 13060, Kuwait. E-mail: f.alsagheer@ku.edu.kw; Tel: +965 24987076
bElectrochemistry and Corrosion Laboratory, National Research Centre, Dokki, Cairo 12622, Egypt
First published on 12th January 2016
Abstract
Herein, we report a facile method for the fabrication of a hydrothermally modified well shaped nanocubed ZnS quantum dot (NCQD)/polyvinyl alcohol nanocomposite. The prepared nanocomposite via this method was characterized using various techniques such as field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA) and atomic force microscopy (AFM). For the first time, a thin film of the fabricated nanocomposite was coated on 316L stainless steel alloy by heat treatment. The corrosion protection efficiency of the PVA/ZnS-NCQD nanocomposite coating on stainless steel was investigated by potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques in 3.5% NaCl solution. The corrosion protection of stainless steel was enhanced in the presence of the PVA/ZnS-NCQD nanocomposite coating which exhibited better corrosion resistance in saline water. The presence of ZnS-NCQDs increases the inhibition efficiency of PVA against the corrosion of stainless steel in chloride solution from 67.0% to 94.0%. The electrochemical corrosion parameters obtained from potentiodynamic polarization and EIS are in good agreement.
Introduction
Corrosion of metals and alloys is a thermodynamically favorable process in which a metal tends to convert to its lower energy oxide form.1–3 But this behavior causes different economic and industrial problems. It is impossible to prevent corrosion of metals therefore research is focusing on slowing down the corrosion kinetics and controlling its mechanism.4 Stainless steels are of great importance in industrial fields because of their corrosion resistance, lower cost, good mechanical properties, excellent fabrication, availability and cheapness compared to other metals.5 There are many problems affecting the mechanical stability and lowering the corrosion resistance of steels including crevice, galvanic and pitting corrosion especially in aggressive media containing chloride.5There are different methods to protect metals from corrosion to extend its working lifetime such as alloying, cathodic protection, passivation, sacrificial metallic coatings and using corrosion inhibitors.6–9 Among the wide variety of corrosion protection techniques, metallic coating considered to be very effective technique. Using the organic–inorganic hybrid coatings is considered one of the promising candidates for environmentally compliant surface protection methods. Nanocomposite coatings have been used effectively in corrosion protection of metals and alloys and considered as an effective alternative for toxic and hazards compounds. It is a multiphase solid material with one phase with size less than 100 nm act as an artificial protective layer on the surface of metal to suppress the cathodic and anodic reactions.10,11
Polymers have attracted considerable interest as anticorrosive materials due to their stability and cost effectiveness.12 They can be adsorbed strongly on the metal surface due to their multiple adsorption sites (functional groups) showing noticed anticorrosion behavior due to formation of complexes with the surface metal ions which occupy a large surface area thereby blanketing the surface and protecting the metal from corrosive agents present in the solution.13,14 One of the most important polymers used as environmentally friendly inhibitor is PVA.15 The coordination of the polymer with metal ions suppresses the mobility of free ions in solution. The conducting polymer coatings polyaniline (PANI) and polypyrrole (PPy), were improved the corrosion resistance of stainless steel and increased its electrical conductivity.16
Degradable polymers are used as anticorrosive coats such as PVA which is biodegradable polymer used as coating for corrosion inhibition several times before.17,18 Those biodegradable polymers have good film formation and eco-friendly. Lee et al., investigated the preparation of PVA/ZrO2-based composite coatings on stainless steel which enhanced its corrosion protection and displayed excellent adhesion and hydrophilicity.17 Also the corrosion current density of 316L stainless steel was decreased using TiN-based coatings and high charge transfer resistance was obtained.18 TiO2/ZrO2 nanocomposite coated over 316L stainless steels was showed a higher corrosion resistance and enhanced biocompatibility.5
In this work we report a simple hydrothermal preparation of PVA/ZnS-NCQDs nanocomposite and its application as a promising coat in protecting 316L stainless steel from corrosion due to chloride attack. To the best of our knowledge, there are no articles has been published for the synthesis of nanocubed ZnS quantum dots and its inclusion as PVA/ZnS-NCQDs nanocomposites coating as anticorrosive candidates for stainless steel in 3.5% NaCl at room temperature. The electrochemical behavior of the coated steel in 3.5% NaCl solution was investigated using potentiodynamic polarization and electrochemical impedance spectroscopy techniques.
Experimental
Chemicals and reagents
All chemicals are AnalaR-grade and were used as received without further purification. Sodium chloride, sodium sulfide, zinc nitrate, and PVA were obtained from Sigma Aldrich (Milwaukee, WI, USA). Aqueous solutions were prepared using de-ionized water and diluted from stock solutions.Preparation of PVP/ZnS-NCQDs
In a typical NCQDs synthesis, 0.2 M zinc nitrate is added to a 5% aqueous solution of PVA with stirring. Then an equal volume of a 0.2 M aqueous solution of Na2S as the sulfur source is added and the resulting solution is allowed to stir till obtaining homogeneity. This mixture is transferred to a 300 mL Teflon lined stainless-steel autoclave and let stand at 110 °C for 5 h.Characterization of PVA/ZnS-NCQDs
The X-ray diffraction (XRD) measurements were conducted by using Bruker diffractometer with copper target and nickel filter with Cu Kα radiation (λ = 0.154056 nm). The morphology of the film was obtained by using scanning electron microscopy (SEM) with a Jeol JEM 1230 operating at 120 kV and Atomic Force Microscope (AFM) Agilent 5420. The average particle size was determined statistically by manually counting 90 particles. X-ray photoelectron spectroscopy (XPS) was conducted using a model VG Scientific 200 spectrometer (UK) equipped with Mg Kα radiation (1253 eV) and operated at 23 kV and 13 mA. Thermogravimetric analysis (TGA) was performed on 10 to 15 mg portion of test materials using a Shimadzu TGA-50 thermogravimetric analyzer (Shimadzu Scientific Instruments, Kyoto, Japan) under nitrogen atmosphere in the temperature range 20 °C to 800 °C with a heating rate of 10 °C min−1.Preparation of thin films
Dip-coating process was used to prepare thin film of nanocomposite by dipping the nanocomposites on the steel surface. Then, the gel films were dried in an oven at 100 °C for 3 min, and this process was repeated up to four times. Then the substrates were thermally treated to 200 °C in a furnace in an air and immediately cooled to room temperature.Electrochemical measurements
The working electrode was prepared from 316L SS alloy with composition of 18% Cr, 14% Ni, 0.03% C, 2.0% Mn, 0.1% Si, 0.45% P, 0.03% S, 3% Mo and the rest iron. Samples for all electrodes have a geometrical area of 1.0 cm2 made of foils 2 mm thick, were embedded in a Teflon holder and were exposed to the solution. The electrodes were polished with fine emery paper of SiC 400 to 1000 grit and with alumina powder down to 0.05 μm followed by ultrasonication in a distilled water bath. A conventional three-electrode cell was used for all electrochemical measurements with a Pt sheet counter electrode having surface area of 1 cm2 and a saturated silver–silver chloride electrode (Ag/AgCl) as a reference electrode.A potentiostat model Gamry PCI4/750 (Gamry, Inc.) was used for electrochemical measurements. The potentiostat was controlled by a PC and the data were analyzed using the Gamry Corrosion Software. Potentiodynamic polarization experiments were performed between an initial potential Ei = −0.5 V before open circuit potential up to a final potential Ef = +0.5 V after open circuit potential at a sweep rate of 1.0 mV s−1; the potential sweep started after a wait period of 20 minutes. Electrochemical impedance spectroscopy measurements were performed at Eapp = 0.0 V with the ac voltage amplitude of 10 mV in the frequency range from 105 to 0.1 Hz. The data analysis software (Echem analyst) was provided with the instrument and applied nonlinear least square fitting with Levenberg–Marquardt algorithm. The surfaces of the electrodes were examined using field-emission scanning electron microscope (FE-SEM) using (JSM-6300 JEOL).
Results and discussion
X-ray diffraction
The X-ray diffraction (XRD) pattern of PVA/ZnS-NCQDs nanocomposite, is shown in Fig. 1, contains various diffraction peaks at 2θ values of 28.7°, 44.7° and 56.6° corresponding to the respective diffraction planes (111), (220) and (311) (ICDD PDF 05-0566).19 The peaks were perfectly indexed to the pure cubic zinc-blende phase of ZnS.19 The average crystallite size (D) was calculated from the full-width at half-maximum (FWHM) using the Debye–Scherrer formula for spherical particles [eqn (1)],
D = 0.89λ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
Surface analysis of ZnS-NCQDs
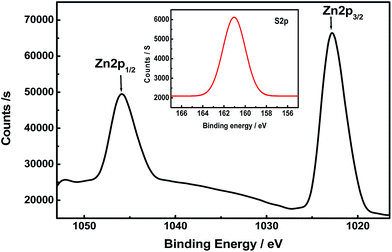
The XPS spectrum of PVA/ZnS-NCQDs nanocomposites is shown in Fig. 2 which illustrates the binding energies of Zn 2p1/2 and 2p3/2 of the prepared sample of ZnS and observed at 1045.0 and 1022.1 eV respectively. The spin–orbit splitting of Zn 2p3/2 and Zn 2p1/2 is 23.0 eV, which is characteristic for ZnS.20 The inset of Fig. 2 shows the S 2p1/2, the binding energy is observed at 162.0 eV which is in accordance with previously reported work.21Thermogravimetric analysis (TGA)
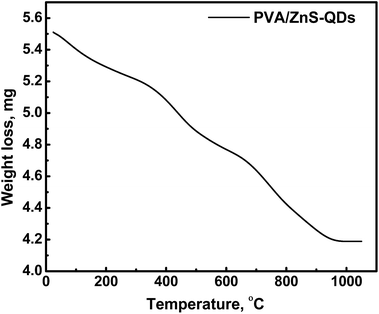
TGA of the PVA/ZnS-NCQDs samples is shown in Fig. 3. Three main decomposition steps can be observed; the first weight loss step is up to about 120 °C, which demonstrating the dehydration of surface-adsorbed water.22 The second step, at 180–430 °C, is related to the decomposition of polymer.23 The third weight loss, at 640 °C, is referred to the decomposition of the remaining adsorbed polymer.24 The thermal behavior of the nanocomposite revealed that it is thermally stable even at elevated temperatures.Electron microscopy
To understand the nature of the SS surface in the absence and presence of the studied coat and the extent of corrosion of stainless steel, the SEM micrographs of the surface are examined. The SEM micrographs of stainless steel surface immersed in the 3.5% NaCl are shown in Fig. 4. It is obvious that for the uncoated SS as shown in Fig. 4A, pits are clearly observed with increasing the roughness of the metal surface which indicates the corrosion of stainless steel in chloride solution. Fig. 4B indicates the PVA/ZnS-NCQDs coated SS surface after immersion in NaCl solution from which it is clear that the nanocubes are monodisperse without any significant aggregation. Also there is no any sign of corrosion under the same experimental conditions, which confirm its efficient protective properties. | ||
| Fig. 4 SEM images for bare SS (A) and coated SS surface scan with different magnifications (B) in 3.5% NaCl solution. | ||
The topography and size distribution of PVA/ZnS-NCQDs nanocomposite was also confirmed by tapping-mode AFM imaging to investigate the location of the ZnS nanoparticles with respect to their PVA coating. 2D AFM images in Fig. 5 exhibited uniform and granular nanoparticles with height distribution (surface root mean square, Sq) of 6.5 nm and average roughness (arithmetic mean height, Sa) of 3.6 nm.
Potentiodynamic polarization
The inhibition efficiency of PVA/ZnS-NCQDs nanocomposite was determined through electrochemical experiments. The potentiodynamic polarization measurements were conducted for stainless steel alone and coated with 1, 2 and 3 layers of PVA/ZnS-NCQDs in 3.5% NaCl solution as shown in Fig. 6 and the parameters are displayed in Table 1. From Fig. 6, sharp decrease was observed in the anodic and cathodic current–voltage curves towards less corrosion current in presence of the investigated coat compared to the bare steel indicating that the corrosion rate was mitigated. Also suggested that the investigated coat presented an inhibiting effect on both anodic dissolution of steel and cathodic hydrogen reduction reaction. The highest corrosion resistance was obtained in case of coated bare steel with three layers of PVA/ZnS-NCQDs nanocomposite. Also it can be seen that the corrosion potential (Ecorr) of the bare steel in 3.5% NaCl solution is −0.241 V which positively shifted to be −0.097 V for the steel coated with three layers of the studied coat which confirms the improvement of the corrosion resistance in presence of the nanocomposite coat. | ||
| Fig. 6 The potentiodynamic polarization curves of the 316L SS alloy alone and coated with PVA/ZnS-NCQDs recorded after 20 minutes of immersion in 3.5% NaCl solution. | ||
| Ecorr, mV | icorr, μA cm−2 | −βc, V dec−1 | βa, V dec−1 | I% | |
|---|---|---|---|---|---|
| Bare SS | −0.241 | 19.5 | 0.260 | 1.03 | — |
| PVA alone | −0.191 | 6.43 | 0.215 | 0.600 | 67.0 |
| One layer of nanocomposite | −0.109 | 3.72 | 0.228 | 0.607 | 80.9 |
| Two layers of nanocomposite | −0.104 | 2.61 | 0.209 | 0.532 | 86.6 |
| Three layers of nanocomposite | −0.097 | 1.17 | 0.137 | 0.446 | 94.0 |
To assess the efficiency of PVA/ZnS-NCQDs nanocomposite to control the steel dissolution, the inhibition efficiency (I) was calculated from polarization curves using the following equation:25
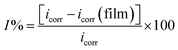 | (2) |
Electrochemical impedance spectroscopy
The electrochemical impedance spectroscopy measurements were performed to better characterize the protective behavior of the newly synthesized PVA/ZnS-NCQDs nanocomposite and to confirm the potentiodynamic polarization results by determining the impedance parameters of the coated steel/electrolyte interface. Fig. 7 displays the Nyquist plots of bare stainless steel and the coated one with 1, 2 and 3 layers of PVA/ZnS-NCQDs in 3.5% NaCl solution with the fitted results. From Fig. 7 it is clear that the diameter of the impedance curve increases in presence of the investigated coat compared with the bare steel and increases more with increasing the layers of the coat. Increasing the diameter of the impedance curve indicates increasing the polarization resistance which confirms increasing the surface resistance of the protective film on the metal surface and suppressing the dissolution of the alloy. | ||
| Fig. 7 The impedance spectra of 316L SS alloy alone and coated with PVA/ZnS-NCQDs recorded after 20 minutes of immersion in 3.5% NaCl solution. | ||
Fig. 8a and b shows the electrical equivalent circuit proposed to model EIS spectra. The different corrosion kinetic parameters derived by fitting the experimental data using the equivalent circuit are listed in Table 2. Fig. 8a represents the data obtained in absence of the studied coat showing one relaxation time constant which represents a single charge transfer reaction. In presence of the studied coat two relaxation time constants were presented as shown in Fig. 8b. Rs is the solution resistance, Rct is the charge transfer resistance, Cdl is the double-layer capacitance, Rc is the resistance for coat and Cc is the capacitance of the coat. To give a more accurate fit, one constant phase element (CPE) is substituted for the capacitive element as the obtained capacitive loop is depressed semicircle rather than regular one due to the roughness and inhomogeneity of the surface.26
| Rs Ω cm2 | Cc μF cm−2 | n1 | Rc Ω cm2 | Cdl μF cm−2 | n2 | Rct kΩ cm2 | I% | |
|---|---|---|---|---|---|---|---|---|
| Bare SS | 3.51 | — | — | — | 43.59 | 0.87 | 1.5 | — |
| PVA alone | 1.48 | 51 | 0.86 | 6.3 | 20.46 | 0.86 | 5.1 | 70.6 |
| One layer of nanocomposite | 3.27 | 46 | 0.87 | 8.7 | 14.62 | 0.87 | 8.7 | 82.8 |
| Two layers of nanocomposite | 4.12 | 39 | 0.89 | 9.6 | 6.77 | 0.89 | 12.5 | 88.0 |
| Three layers of nanocomposite | 2.88 | 31 | 0.84 | 15.1 | 1.57 | 0.88 | 45.5 | 96.7 |
The impedance of CPE is described by the expression:27
| ZCPE = Yo−1(jω)−n | (3) |
The correction of the capacity to its real value is calculated according to Hsu and Mansfeld,27 as follow:
| Cdl = Yo(ωmax)n−1 | (4) |
From Table 2, the Cdl value obtained is higher in case of the bare steel in chloride solution (43.59 μF cm−2). In presence of one layer of PVA/ZnS-NCQDs coated steel, Cdl significantly decreases from 43.59 to 14.62 μF cm−2. By increasing the layers of the investigated coat more pronounced decease in Cdl value obtained reaching 1.57 μF cm−2 in presence of three layers of the coat. The decrease of Cdl values in presence of the coat is probably due to an increase in the thickness of the electrical double layer and/or a decrease in the local dielectric constant suggesting the strong attachment of the coat to the steel surface.28 Also, the charge transfer resistance (Rct) increases in the presence of PVA/ZnS-NCQDs nanocomposite with maximum increase in presence of three layers of the coat from 1.5 for the bare 316L SS to 45.5 kΩ cm2. To calculate inhibition efficiency from the Rct the following eqn (5) was used.
| I% = [(Rct − Rct°)/Rct] × 100 | (5) |
Fig. 9a and b, shows the Bode impedance magnitude and phase angle plots recorded for bare stainless steel electrode alone and coated with different layers of PVA/ZnS-NCQDs nanocomposite in 3.5% NaCl solution at open circuit potential. The impedance plots at higher frequency limit (100 kHz) correspond to the ohmic resistance of the films of corrosion product and the solution between the working electrode and reference electrode. An ideal capacitive behavior would be the result if a phase angle value attains −90° and a slope value attains −1. The slopes of Bode impedance magnitude plots at intermediate frequencies, S, and the maximum phase angles, α, showed deviation from the values of −1 and 90°, respectively. These deviations represent the deviation from the ideal capacitive behavior at intermediate frequencies. The Bode phase angle plots show single maximum at intermediate frequencies, broadening of this maximum in presence of PVA/ZnS-NCQDs account for the formation of a protective layer on electrode surface. For imperfect coating, the underlying metal comes in contact with the solution, and hence Rct and Cdl of the metal surface also need to be included in the equivalent circuit which explains the probability of presence of two time constants. The addition of ZnS-NCQDs increases the impedance values than PVA alone and improves the corrosion resistance of the nanocomposite. This improvement related to the acceleration of the chemical passivation and thickening of the film which increases the protection of steel surface by blocking the active sites on its surface.
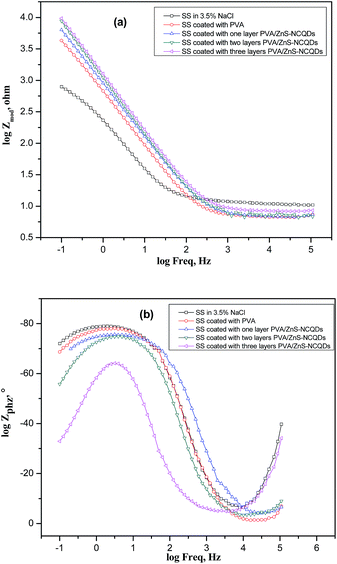 | ||
| Fig. 9 Bode plots for the 316L SS alloy alone and coated with PVA/ZnS-NCQDs recorded after 20 minutes of immersion in 3.5% NaCl solution. | ||
Mechanism of superior protection
ZnS is embedded within the polymer matrix as nanoparticulated solid that is dispersed in coating material to provide certain characteristics to it such as durability, mechanical strength and corrosion protection for metallic substrates.29 Also, ZnS is a stable semiconductor material with a wide band gap Eg = 3.8 eV, and it can also form a potential barrier at the electrode–electrolyte interface due to its more negative conduction band (1.8 V vs. NHE).30 Also, introducing ZnS-NCQDs in the nanocomposite increases the surface area of nanocomposite and this leads to increasing the surface area of the surface coat; this can increase the ability of the PVA/ZnS-NCQDs nanocomposite coating to interact with the ions liberated during corrosion reaction and/or it causes an increase in the rate probability for the occurrence of cathodic reduction of oxygen and enhances effectively the polymer oxidation and the formation of the oxide film.Also the superior protection for the incorporation of ZnS QDs is most likely originated from its cubic shape. As it is well known that both morphology and size of nanoparticles are key determining factor for specific applications. As the adhesion in the coating application is the key factor for good candidates of protective coatings. Particularly, non-spherical shapes are preferred where a larger contact area can lead to more-robust binding.31 Thus, nanocubes could be the good candidate comparing with other shapes because of its large contact area which can increase its adhesion capability. Therefore, integration of the cubic structure of ZnS QDs obviously enhances PVA performance and thus becomes a promising material for coating applications.
The mechanism of the enhanced corrosion protection of the PVA/ZnS-NCQDs nanocomposite coating might be a result of the well-dispersed ZnS-NCQDs in the PVA matrix that increases the resistance of steel to corrosion by forming a passive film at the metal polymer interface. There are different steps of oxidation and reduction occurs to steel during corrosion and rust formation as follow:32
| Fe → Fe2+ + 2e− | (6) |
| Fe2+ → Fe3+ + e− | (7) |
| O2 + 2H2O + 4e− → 4OH− | (8) |
| 2Fe2+ + O2 (g) + 2H2O → 2FeOOH + 2H+ | (9) |
The presence of sufficient H2O and O2 is important for rust formation and steel corrosion so, suppressing any of these reactions will overcome the corrosion and enhance the steel inhibition which well achieved using coating. Therefore, it is reasonable to believe that the presence of PVA/ZnS-NCQDs nanocomposite coating is able to effectively prevent the H2O and O2 from accessing the substrate surface leading to a good anticorrosion property. In addition, the incorporated ZnS-NCQDs also enhance the passivation of the coated SS in chloride solution.
Conclusions
In this study, PVA/ZnS-NCQDs nanocomposite was successfully prepared and treated hydrothermally inside stainless steel autoclave. The synthesized nanocomposite was utilized for metal protection targets as anticorrosive. The waterborne PVA/ZnS-NCQDs coat was prepared and applied on stainless steel by dip coating method. The potentiodynamic polarization confirmed the lower current density and nobler corrosion potential in case of the stainless steel coated with PVA/ZnS-NCQDs nanocomposite compared to uncoated stainless steel in 3.5% NaCl solution. The presence of three layers of the coat showed remarkable increase in the inhibition efficiency up to 94.0%. EIS plots indicated that the charge transfer resistance increases in presence of the investigated coat which confirms increasing the corrosion resistance of steel. In conclusion, PVA/ZnS-NCQDs nanocomposite is an efficient environmental material for the protection of the stainless steel.Acknowledgements
The authors gratefully acknowledge the support of Kuwait University Research administration, SAF Facilities No. (GS 03/01, GE 01/07, GE 03/08 and GS 02/08).Notes and references
- A. F. Frau, R. B. Pernites and R. C. Advincula, Ind. Eng. Chem. Res., 2010, 49, 9789–9797 CrossRef CAS.
- G. X. Shen, Y. C. Chen and C. J. Lin, Thin Solid Films, 2005, 489, 130–136 CrossRef CAS.
- H. Yun, J. Li, H.-B. Chen and C.-J. Lin, Electrochim. Acta, 2007, 52, 6679–6685 CrossRef CAS.
- A. C. de Leon, R. B. Pernites and R. C. Advincula, ACS Appl. Mater. Interfaces, 2012, 4, 3169–3176 CAS.
- S. Nagarajan, M. Mohana, P. Sudhagar, V. Raman, T. Nishimura, S. Kim, Y. S. Kang and N. Rajendran, ACS Appl. Mater. Interfaces, 2012, 4, 5134–5141 CAS.
- A. Abdel Nazeer, N. K. Allam, G. I. Youssef and E. A. Ashour, Ind. Eng. Chem. Res., 2011, 50, 8796–8802 CrossRef CAS.
- A. Abdel Nazeer, H. M. El-Abbasy and A. S. Fouda, J. Mater. Eng. Perform., 2013, 22, 2314–2322 CAS.
- A. Abdel Nazeer, N. K. Allam, A. S. Fouda and E. A. Ashour, Mater. Chem. Phys., 2012, 136, 1–9 CrossRef.
- C. Suryanarayana, K. C. Rao and D. Kumar, Prog. Org. Coat., 2008, 63, 72–78 CrossRef CAS.
- J. Carneiro, J. Tedim, S. C. M. Fernandes, C. S. R. Freire, A. Gandini, M. G. S. Ferreira and M. L. Zheludkevich, Surf. Coat. Technol., 2013, 226, 51–59 CrossRef CAS.
- S. A. S. Dias, S. V. Lamaka, C. A. Nogueira, T. C. Diamantino and M. G. S. Ferreira, Corros. Sci., 2012, 62, 153–162 CrossRef CAS.
- M. Srimathi, R. Rajalakshmi and S. Subhashini, Arabian J. Chem., 2014, 7, 647–656 CrossRef CAS.
- B. P. Singh, B. K. Jena, S. Bhattacharjee and L. Besra, Surf. Coat. Technol., 2013, 232, 475–481 CrossRef CAS.
- S. Rajendran, S. P. Sridevi, N. Anthony, A. A. John and M. Sundearavadivelu, Anti-Corros. Methods Mater., 2005, 52, 102–107 CrossRef CAS.
- M. Chaouat, C. Le Visage, W. E. Baille, B. Escoubet, F. Chaubet, M. A. Mateescu and D. Letourneur, Adv. Funct. Mater., 2008, 18, 2855–2861 CrossRef CAS.
- N. D. Nam, J. G. Kim, Y. J. Lee and Y. K. Son, Corros. Sci., 2009, 51, 3007–3013 CrossRef CAS.
- P.-J. Lee, C.-C. Ho, C.-S. Hwang and S.-J. Ding, Surf. Coat. Technol., 2014, 258, 374–380 CrossRef CAS.
- M. Li, S. Luo, C. Zeng, J. Shen, H. Lin and C. Cao, Corros. Sci., 2004, 46(6), 1369–1380 CrossRef CAS.
- D. W. Synnott, M. K. Seery, S. J. Hinder, G. Michlits and S. C. Pillai, Appl. Catal., B, 2013, 130–131, 106–111 CrossRef CAS.
- S. A. Acharya, N. Maheshwari, L. Tatikondewar, A. Kshirsagar and S. K. Kulkarni, Cryst. Growth Des., 2013, 13, 1369–1376 CAS.
- M. H. Ullah, I. Kim and C.-S. Ha, Mater. Lett., 2007, 61, 4267–4271 CrossRef CAS.
- R. Viswanath, H. S. B. Naik, Y. K. G. Somalanaik, P. K. P. Neelanjeneallu, K. N. Harish and M. C. Prabhakara, J. Nanotechnol., 2014, 2014, 924797–924804 Search PubMed.
- H. Al-Maydama, A. El-Shekeil, M. A. Khalid and A. Al-Karbouly, Ecletica Quim., 2006, 31, 45–52 CAS.
- A. Tena, S. Shishatskiy and V. Filiz, RSC Adv., 2015, 5, 22310–22318 RSC.
- A. Abdel Nazeer, E. A. Ashour and N. K. Allam, Mater. Chem. Phys., 2014, 144, 55–65 CrossRef.
- X. Wu, H. Ma, S. Chen, Z. Xu and A. Sui, J. Electrochem. Soc., 1999, 146, 1847–1853 CrossRef CAS.
- C. H. Hsu and F. Mansfeld, Corrosion, 2001, 57, 747–749 CrossRef CAS.
- A. Abdel Nazeer, H. M. El-Abbasy and A. S. Fouda, Res. Chem. Intermed., 2013, 39, 921–930 CrossRef CAS.
- R. Talbert, Paint Technology Handbook, Taylor & Francis Group, London, 2008 Search PubMed.
- Y.-F. Zhu, J. Zhang, L. Xu, Y. Guo, X.-P. Wang, R.-G. Du and C.-J. Lin, Phys. Chem. Chem. Phys., 2013, 15, 4041–4048 RSC.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4(3), 310–325 CrossRef CAS.
- C. H. Chang, T. C. Huang, C. W. Peng, T. C. Yeh, H. I. Lu, W. I. Hung, C. J. Weng, T. I. Yang and J. M. Yeh, Carbon, 2012, 50, 5044–5051 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |