A facile one-step route to synthesize the three-layer nanostructure of CuS/RGO/Ni3S2 and its high electrochemical performance†
Abstract
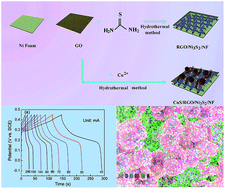
A three-layer nanostructure of a CuS/reduced graphene oxide (RGO)/Ni3S2 composite was in situ grown on nickel foam (NF) through a one-step hydrothermal-assisted process. During this process, the bottom Ni3S2 layer and middle RGO layer were simultaneously formed through the redox reaction of the Ni element on the foam surface of NF with GO and subsequent vulcanization. The upper CuS layer, consisting of sphere and fiber-like blocks, was converted from Cu2+ adsorbed by electrostatic forces and then well anchored on the RGO surface. The binder-free CuS/RGO/Ni3S2 electrode delivered high specific capacitance (10 494.5 mF cm−2 at a current density of 40 mA cm−2, i.e., 1692.7 F g−1 at 6.5 A g−1). It also exhibited an excellent cycling stability with ca. 91.5% of the initial capacitance retention after 4000 charge–discharge cycles at a current density of 100 mA cm−2. The good electrochemical performance and simple accessibility prove that this CuS/RGO/Ni3S2 composite is a promising material for supercapacitor applications.

 Please wait while we load your content...
Please wait while we load your content...