Enhanced microwave absorption properties of ferroferric oxide/graphene composites with a controllable microstructure†
Rui Zhanga,
Xiaoxiao Huang*a,
Bo Zhongb,
Long Xiab,
Guangwu Wenab and
Yu Zhoua
aSchool of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China. E-mail: swliza@hit.edu.cn; Fax: +86-451-86413922; Tel: +86-451-86418694
bSchool of Materials Science and Engineering, Harbin Institute of Technology at Weihai, Weihai, 264209, China
First published on 4th February 2016
Abstract
Fe3O4/graphene composites were synthesized as an advanced electromagnetic wave absorption material by a solvothermal method in a system of ethylene glycol. The Fe3O4 nanoparticles were homogeneously anchored on the graphene sheets and the structures of the nanoparticles could be experimentally controlled from ring-like spheres, flower-like spheres to solid spheres by changing the concentration of the oxide graphene. Microwave absorption tests demonstrated that the structures of the nanoparticles had a positive influence on the microwave absorption properties. Especially, for the Fe3O4/graphene composite with a flower-like structure, the minimum reflection loss value (RL) could reach −53.2 dB and the bandwidth of RL less than 10 dB (90% absorption) ranged from 8.1 to 16 GHz at a thickness of 2.5 mm, which is among the best-reported performances of Fe3O4/graphene materials, showing a huge potential to be used as a candidate for microwave absorbing materials.
Introduction
In recent decades, electromagnetic wave absorption (EMA) materials have received much attention due to the urgent requirements in the fields of electrical apparatus, information technology, human health1 and military equipment.2 Generally, EMA materials can be classified into two kinds: one is dielectric loss materials like carbon nanotubes and conductive polymers,3–5 the other is magnetic loss materials such as Ba M-type ferrites,6 Ni, and so on. Among the latter, ferroferric oxide (Fe3O4) nanoparticles, one of the typical ferromagnetic materials, is considered as an ideal microwave absorption material due to the low cost, excellent magnetic properties, non-toxicity, high compatibility and morphology controllability.7,8 However, the use of nanoparticles in microwave absorption has some limitations, typically related to the high density and easy agglomeration. In order to improve the EMA properties, a variety of strategies as following have been applied to the Fe3O4 nanoparticles: (1) the Fe3O4 nanoparticles coated with graphitic or non-graphitic carbon (carbon,9 carbon nanohorns10 and graphene11,12); (2) Fe3O4 based composite architectures (other metal oxide13 and conductive macromolecule14); (3) unique Fe3O4 nanostructure/microstructure with hollow hemispheres,15 bowl like16 and porous hollow beads.17 In these strategies mentioned above, Fe3O4/graphene composites have been developed to provide outstanding electromagnetic property.Graphene, a newfound carbon material with the structure of sp2 carbon atoms tightly packed into a honeycomb network, has been regarded as an ideal component of the EMA materials due to its large specific surface area (2630 m2 g−1), excellent electronic mobility (2 × 105 cm2 V−1 s−1), thermal conductivity (5000 W m−1 K−1), and mechanical strength (Yong's modulus, 1100 GPa).18 According to the previous reports about microwave absorbers, many effective efforts have been focused on compositing graphene with nitrile butadiene rubber,19 Ni,20,21 Co3O4,22 SiO2,23 macromolecule,24 polyaniline–gold25 and so on. In these composites, the ultrathin flexible graphene sheets can provide a substrate to anchor the nanoparticles, and prevent the agglomeration of nanoparticles. Besides, the good impedance match characteristic also contributes to improve their EMA properties. Therefore, graphene-based magnetic hybrids are believed to be an ideal component in the EMA materials. Previous literatures26–28 about the Fe3O4/graphene (or reduced graphene oxide) composites also indicated that the EMA properties have been improved compared with Fe3O4 nanoparticles or graphene alone. For example, Hu et al.29 reported the 3D graphene–Fe3O4 composites exhibited the calculated minimum RL about −27 GHz with a thickness of 2 mm; Li et al.28 exhibited the maximum reflection loss value of −30.1 dB at a 1.48 mm and 17.2 GHz matching frequency. But all of them researched only on one single morphology of the nanoparticles, and the researches about the morphology controllability of Fe3O4 nanoparticles as well as the effect of the nanostructures on the EMA properties are rare.
In this article, Fe3O4/graphene composites are fabricated by a facile solvothermal method. The Fe3O4 nanoparticles with different size, bearing capacities and morphologies are uniformly spread over graphene sheets, and the influence of the controllable morphology on the EMA properties has been systematically studied. Consequently, through changing the mass ratio of the raw materials, the morphology of Fe3O4 nanoparticles could be easily controlled and changed from the ring-like spheres, flower-like spheres to solid spheres. Specially, the composite with flower-like particles which shows the optimum properties of high absorbing intensity, wide absorption frequency, thin thickness and light density. The outstanding properties highlight the great potential of Fe3O4/graphene composites as promising materials for lightweight and high performance absorbers.
Experimental
Fabrication of the Fe3O4/graphene composites
The graphene oxide (GO) was synthesized through a modified Hummer's method.30 The Fe3O4/graphene composites were fabricated in ethylene glycol. In a typical experiment, the ground GO powder was dissolved in 30 ml ethylene glycol (concluding 0.75 ml deionized water) with ultrasonic dispersion for 3 hours, and then 0.54 g FeCl3·6H2O and 1.05 g urea were added into the solution and mixed uniformly. Next, the solution was transferred into a 50 ml Teflon-lined autoclave and maintained at 200 °C for 14 h. Subsequently, the products were washed with deionized water and ethyl alcohol by centrifugation. Fe3O4/graphene composites with different mass ratio of GO and Fe3O4 nanoparticles (MGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MFe3O4) were obtained and labeled as sample 1 (1
MFe3O4) were obtained and labeled as sample 1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), sample 2 (5
1), sample 2 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), sample 3 (10
1), sample 3 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and sample 4 (20
1) and sample 4 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The pure Fe3O4 nanoparticles are synthesized with the same method without GO.
1). The pure Fe3O4 nanoparticles are synthesized with the same method without GO.
Characterization
The morphologies of the as-synthesized samples were characterized by the scanning electronic microscopy (SEM, FEI-Quanta 200F), and transmission electron microscopy (TEM, JEOL210 – FEI Tecnai G2 F30 at 300 KV). The powder X-ray diffraction (XRD) patterns were recorded on Rigaku D/max-γ B X-ray diffractometer using Cu Kα radiation. X-ray photoelectron spectroscopy (XPS) studies were performed using the Thermo Fisher 0ESCALAB 250Xi. Raman studies of randomly oriented samples were performed with a Renishaw Ramoscope (Confocal Raman Microscope, in Via, Renishaw). The magnetic hysteresis loops were performed by a vibrating sample magnetometer (VSM, SQUID-VSM) at room temperature. Thermal gravimetric analysis was performed on a thermal analyzer (Mettler-Toledo TGA/SDTA851e) from room temperature to 800 °C in air at a heating rate of 10 °C min−1. The electromagnetic parameters of complex magnetization and complex permeability were investigated by Vector Network Analyzer, Agilent, N5230A. The samples were prepared by evenly mixing the product with a paraffin max in mass fraction of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and then being pressed into a toroidal-shaped special stainless steel mould with the inner and outside diameters of 3 and 7 mm, respectively.
4 and then being pressed into a toroidal-shaped special stainless steel mould with the inner and outside diameters of 3 and 7 mm, respectively.
Results and discussion
Microstructure, chemical analysis and morphology of Fe3O4/graphene composites
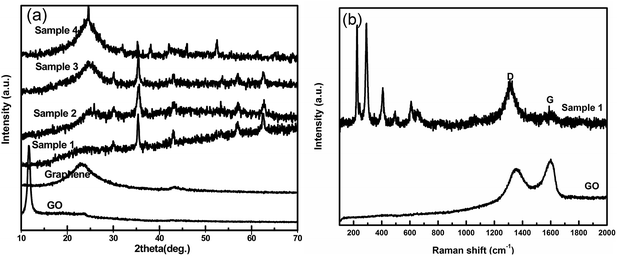
Fig. 1a shows the XRD of the GO, graphene and Fe3O4/graphene composites. Because of the oxidation, the GO shows a sharp diffraction peak at 11.6°, corresponding to a larger basal spacing of 0.74 nm. The characteristic diffraction peak of graphene reduced by ethylene glycol presents a weak and broad diffraction peak approximately at 24°, which proves the removing of oxygen-containing groups and disordered stacking of single graphitic sheets. A cubic structure phase for Fe3O4 in the composites is confirmed by XRD with well-defined diffraction peaks at pattern of 30.1, 35.45, 43.1, 53.5, 56.9 and 62.57° corresponding to (220), (311), (400), (422), (511) and (440) lattice planes (JCPD card no. 65-3107) without any impurity phases (Fig. 1a and S1†). Besides, because of the overlapping of graphitic layers, the peaks become weaker for Fe3O4 nanoparticles while sharper for the graphite from sample 1 to sample 4 shown in Fig. 1a. It indicates that the nanoparticles could prevent the stacking of carbon layers. Raman spectra analysis shown in Fig. 1b further indicates the reduction of the GO and the appearance of the Fe3O4 particles in the composites. Raman spectrum of GO and sample 1 display a prominent G-band (1599 cm−1) along with D-band (1352 cm−1), and the D/G intensity ratio increases from 0.89 for GO to 1.26 for sample 1. This means that the decrease in the average size of the sp2 domains31 and the removing of most oxygen-containing groups. Additionally, the Raman bands for sample 1 at 224.6 and 493.1 cm−1 correspond to the A1g mode and the bands at 291.4, 406.9 and 608.3 cm−1 could be attributed to the Eg mode of Fe3O4, which are consistent with the results in XRD patterns.To investigate the chemical compositions of Fe3O4/graphene composites, XPS were carried out as shown in Fig. 2. In the XPS spectra of sample 1, the C1s peak at 284.7 eV contains three kinds of components, arising from C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the graphitic carbon, C–O in the residual oxygen-containing groups and C–N due to the N doped on the graphitic layers, respectively32 (Fig. 2b). The O1s peak at 531.3 eV can be fitted to four peaks (O–C–O, Fe–O, Fe–O–C, and C–O peak), which mainly come from the Fe3O4 and residual oxygen groups (Fig. 2c). Noteworthily, the Fe–O–C bond at 530.3 eV proves the strong chemical connection between the Fe3O4 and graphitic carbon. Furthermore, two peaks at 711.1 eV and 724.8 eV corresponding to the band energies of Fe2p3/2 and Fe2p1/2, respectively, indicate the formation of a mixed oxide of Fe(II) and Fe(III), and further confirm the existence of Fe3O4 nanoparticles.
C in the graphitic carbon, C–O in the residual oxygen-containing groups and C–N due to the N doped on the graphitic layers, respectively32 (Fig. 2b). The O1s peak at 531.3 eV can be fitted to four peaks (O–C–O, Fe–O, Fe–O–C, and C–O peak), which mainly come from the Fe3O4 and residual oxygen groups (Fig. 2c). Noteworthily, the Fe–O–C bond at 530.3 eV proves the strong chemical connection between the Fe3O4 and graphitic carbon. Furthermore, two peaks at 711.1 eV and 724.8 eV corresponding to the band energies of Fe2p3/2 and Fe2p1/2, respectively, indicate the formation of a mixed oxide of Fe(II) and Fe(III), and further confirm the existence of Fe3O4 nanoparticles.
SEM and TEM are used to investigate the microstructure of the products. Fig. S2a† is the representative view of the graphene with a crumple structure. The ring-like spheres Fe3O4 nanoparticles with an average diameter of 200 nm and a shell thickness of 50 nm are uniformly deposited on the graphitic sheets in sample 1 as shown in Fig. 3a and b. They are different from the pure Fe3O4 nanoparticles which present hollow structures with a diameter of about 200 nm and a shell thickness of 50 nm (Fig. S2(b) and S3–S5†). Fig. 3b further indicates that all the Fe3O4 nanoparticles are homogeneously embedded on the wrinkle graphene sheets. The HRTEM image and the SAED pattern presented in the Fig. 3c clearly demonstrate the single crystalline structure of Fe3O4 nanoparticles in the composites.
 | ||
| Fig. 3 (a) SEM, (b) (c) TEM (d) HRTEM images of the composites sample 1, the inset of (c) shows the SAED pattern of sample 1. | ||
The sample 2 and sample 3 present distinct flower-like spheres with a size of about 50 nm in Fig. 4. More nanoparticles are assembled on the graphene sheets in sample 2 than sample 3 (Fig. 4a–d). This means it is easy to control the loading amount and morphology of nanoparticles on the graphene sheets by altering the mass ratio of reagents. Fig. 4e and f present the TEM and HRTEM images of the flower-like composites. The uniform Fe3O4 nanoparticles are deeply and firmly embedded on the graphene sheets. As shown in Fig. 4f, the crystal lattice fringes with a spacing of 0.253 nm and 0.297 nm are assigned to the (311) and (220) plane of the Fe3O4 crystal, respectively, which are consistent with the results of Fig. 1a. Besides, the small cluster of the Fe3O4 is constructed with many small Fe3O4 nanoparticles with diameters ranging from 10 to 25 nm. Fig. S6† shows the morphology of the composites sample 4 with solid Fe3O4 sphere. Few Fe3O4 nanoparticles are anchored on the carbon sheets because of the huge mass ratio, and this also indicates the controllability of the loading amount and morphology of nanoparticles.
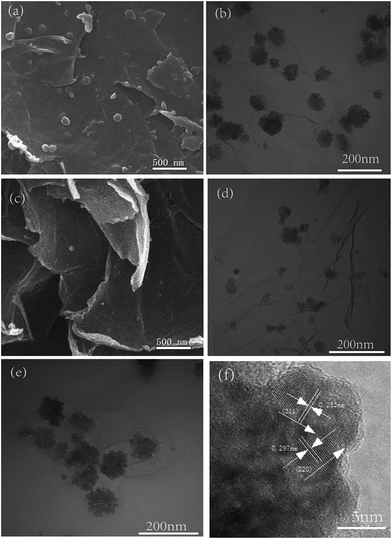 | ||
| Fig. 4 (a) SEM and (b) TEM image of sample 2, (c) SEM and (d) TEM image of sample 3, (e) TEM and (f) HRTEM images of the flower-like composites. | ||
Several characteristics of the composites should be mentioned: (1) it is easy to control the morphology of the nanoparticles from the ring-like sphere, flower-like structure to the solid sphere with the increase of reactants ratio; (2) the nanoparticles firmly attached on the carbon layers after a powerful sonication treatment during the preparation of TEM samples. This shows the strong connection between the carbon and the nanoparticles, which are consistent with the results of XPS; (3) it is noteworthy that the flower-like nanoparticles on graphene sheets have smaller size and more interfaces than the ring-like spheres in sample 1 or the solid spheres in sample 4, which may have strong influence on the EMA properties due to the interface loss.
Fig. 5 shows the possible evolution process of Fe3O4/graphene composites. The GO can attract the Fe3+ and Fe2+ due to the electrostatic interaction between the oxygen-containing groups and iron ions. In this process, some ferric ions connect with the oxygen-containing groups linked to the carbon layers, the others connect with the free oxygen-containing groups in the solution. Accordingly, two kinds of Fe3O4 nanoparticles are fabricated, as shown in Fig. 5a. However, the growth of the two kinds of nanoparticles is not synchronous: the nanoparticles which are not linked on the graphitic layer grow up sharply, while the growth of the nanoparticles connected on the carbon layers is limited. Meanwhile, with increasing time, the reduction of the GO and the growth of Fe3O4 happened simultaneously (Fig. S7†). Consequently, the Fe3O4/graphene composites are fabricated by the easy solvothermal process.
 | ||
| Fig. 5 (a) Schematic view of the procedure of the as prepared Fe3O4/graphene composite; the illustration of the composites fabrication process (b) sample 1, (c) sample 2 and sample 3, (d) sample 4. | ||
Based on the analysis above, the possible fabricated processes are shown in the Fig. 5b–d. In sample 1 plentiful iron ions can unite together to form spheres and then evolve into ring-like structures driven by the minimization of interfacial energy and magnetic dipole interaction (shown in Fig. 5b). In sample 2 and sample 3, the quantity of the oxygen-containing groups increases compared with sample 1, and the interactions between the ions and these groups are stronger than those between ions themselves. In another words, the excess groups restrict the growth and the self-assembly of Fe3O4 nanoparticles, which can only combine with some nearby small size atoms to form flower-like structure. Moreover, with further increasing of the GO mass ratio, the limited iron ions could just assemble on the edges and full defects areas and then form into solid spheres as shown in Fig. 5d.
The magnetic properties of the composites and hollow Fe3O4 nanoparticles were examined at 300 K shown in Fig. 6a. The magnetic hysteresis loops of the pure Fe3O4 nanoparticles and sample 1, 2 and 3 composites show S-like shape to the curve and are ferromagnetic. The saturation magnetization values of these composites of are 37.1 (sample 1), 10.4 (sample 2), 4.7 (sample 3) emu g−1, respectively, while that of hollow Fe3O4 nanoparticles is 79 emu g−1. It indicates the saturation magnetization decreases with the increase of the mass ratio of graphene content. In sample 4, there is almost no magnetism because of the low levels of the Fe3O4 nanoparticles. And from Fig. 6a, it indicates the magnetization of the composites is mainly related to the mass ratio of Fe3O4, and has little relationship with the morphology. Fig. 6b shows the TG curves of the products with different mass ratio. Thermograms of the composites show two major weight losses. The first is at about 100 °C which is due to the loss of water. The second is in the range of 370 to 500 °C and indicates the oxidization of graphene to CO2 and the Fe3O4 to Fe2O3 in air.11 When the temperature reached 800 °C, the residue is Fe2O3 for all the composites. And the calculated Fe3O4 contents derived from the TG data are 51.71, 20.269, 13.6 and 7.39 wt% for the corresponding graphene to Fe3O4 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 20
1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
 | ||
| Fig. 6 (a) Magnetization curves at room temperature of the pure Fe3O4 and Fe3O4/graphene composites; (b) the TGA curves of prepared Fe3O4/graphene composite. | ||
Microwave absorption properties of Fe3O4/graphene composites
To evaluate the EMA properties, the complex permittivity and permeability of Fe3O4 nanoparticles, graphene, and Fe3O4/graphene composites are measured in the range of 2–18 GHz as shown in Fig. 7.The real parts of permittivity (ε′) of graphene and composites (sample 1, sample 2, sample 3, sample 4) decline from 7.07 to 4.11, 10.10 to 4.96, 11.19 to 5.6, 10.62 to 5.6 and 9.8 to 4.8 with the increasing frequency, respectively, while ε′ for pure Fe3O4 nanoparticles indicates negligible change showing little dielectric properties.33,34 It is known that the ε′ is associated with the amount of polarization in the absorber which mainly contain the dipolar polarization and interfacial polarization. And both these two kinds of polarization mechanisms are more frequency-dependent. So the decrease of the ε′ for the composites may due to the decrease in space charge polarization with increasing frequency.35 Additionally, ε′ increases with the increase of mass ration from sample 1 to sample 3 but drops for sample 4, while all the composites have a higher value of ε′ than Fe3O4 which may be caused by the different synergistic effect between the graphene and Fe3O4 nanoparticles. As shown in the Fig. 7b, the imaginary parts of complex permittivity (ε′′) exhibit a peak in the range of 10–17 GHz for the composites, indicating a resonance behavior. While ε′′ for pure Fe3O4 nanoparticles maintains a low value and almost unchanged with increasing frequency. Because the complex permeability contributes to the dielectric loss property,36 the value of ε′′ of the composites and graphene is higher than pure Fe3O4 nanoparticles, which suggests that the electronic spin and charge polarization from the polarized centers have a great effect on the EMA properties of the composites.32 And the values of ε′′ of sample 2 and 3 are almost the highest, which may due to the flower-like morphology. The real parts and imaginary parts of complex permeability (μ′ and μ′′) of the Fe3O4 nanoparticles, graphene and composites, are shown in Fig. 7c and d. The values of μ′ fluctuate with a peak at 16 GHz for all the composites, may be caused by the eddy current effect. The imaginary parts of complex permeability (μ′′) also fluctuate around −0.2 to 0.2 in the range of 2–18 GHz, indicating the surface effect and spin wave excitations. Moreover, the value of μ′′ exhibiting negative values under high frequencies attributes to the motion of charges, which would generate an alternating electromagnetic field.5
Generally, Debye dipolar relaxation has an important influence on the EMA properties of dielectric absorbing materials. The relative complex permittivity can be expressed by the following equation,37
 | (1) |
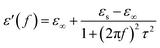 | (2) |
 | (3) |
The relationship between ε′ and ε′′ can be obtained from eqn (2) and (3),
| (ε′ − ε∞)2 + (ε′′)2 = (εs − ε∞)2 | (4) |
Thus, the plot of ε′ versus ε′′ would represent a single semicircle, generally denoted as the Cole–Cole semicircle, which corresponds to one Debye relaxation.
Fig. 8 shows the ε′–ε′′ curve of pure Fe3O4 nanoparticles, graphene and composites. Two semicircles can be observed obviously in the ε′–ε′′ curve of the composites (sample 1 to sample 4), while just an inconspicuous semicircle can be found in the pure Fe3O4 nanoparticles. This indicates that the graphene enhances the dielectric properties of the composites due to the double relaxation mechanism.3 As previously mentioned in XPS and Raman analyses, there are many residual oxygen-containing groups and defects (such as lacking of carbon atoms, polyaromatic islands38) on the graphitic sheets, which can act as the polarization center. Additionally, the interfacial polarization between the nanoparticles and the graphitic sheets also enhances the microwave loss.39 So the increased relaxations for the composites compared with the pure Fe3O4, may arise from the interfacial polarizations between the nanoparticles and the graphene sheets, as well as the dipole polarizations induced by the residual oxygen-containing groups and defects.40 Fig. 8b and c show the dielectric loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε = ε′′/ε′) and magnetic loss factor (tan
δε = ε′′/ε′) and magnetic loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ = μ′′/μ′) of the composites with different mass ratio. The values of tan
δμ = μ′′/μ′) of the composites with different mass ratio. The values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε increase from 2 to 16 GHz while decreases during higher frequencies for all the composites. Meanwhile, the composites have higher tan
δε increase from 2 to 16 GHz while decreases during higher frequencies for all the composites. Meanwhile, the composites have higher tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε value than pure Fe3O4 nanoparticles, indicating a higher dielectric loss. Moreover, the values of tan
δε value than pure Fe3O4 nanoparticles, indicating a higher dielectric loss. Moreover, the values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ exhibit nonlinear change with increasing frequency. Obviously, the value of tan
δμ exhibit nonlinear change with increasing frequency. Obviously, the value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε is higher than that for the tan
δε is higher than that for the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ, which means the dielectric loss play a greater role in the reflection loss of the Fe3O4/graphene composites.
δμ, which means the dielectric loss play a greater role in the reflection loss of the Fe3O4/graphene composites.
 | ||
| Fig. 8 (a) Typical Cole–Cole curves (b) dielectric loss factor and (c) magnetic loss factor of Fe3O4 nanoparticles, graphene and Fe3O4/graphene composites. | ||
According to the transmission line theory, the reflection loss (RL) of all products are calculated by the model of single absorber layer:41
 | (5) |
 | (6) |
Fig. 9 show the calculated RL curves of Fe3O4 nanoparticles, graphene and Fe3O4/graphene composites with different thicknesses in the range of 2–18 GHz. The pure Fe3O4 nanoparticles with a hollow structure have a weak RL value which does not exceed −10 dB (shown in Fig. 9a). While the graphene reduced from GO by ethylene glycol shows a good absorption for microwave with a minimum RL value of −35 dB at the thickness of 2 mm, which is different from the general result.42 The interesting RL value of the graphene in our work may be caused by the residual oxygen-containing groups which can act as polarization center and so generate the polarization relaxation.42 Fig. 9c shows the minimum RL value of −23.7 dB at 15.3 GHz (d = 2.5 mm) and the bandwidth (RL < −10 dB) is about 6 GHz for sample 1. It is also observed that the absorption peak shifts to the low frequency with the increase of absorber thickness corresponding to the quarter-wave principle.28 Remarkably, sample 2 with the flower-like structure has the minimum RL of −53.2 dB at 11.4 GHz (d = 2.5 mm) and the bandwidth of RL < −10 dB about 7 GHz which shows a better EMA properties than sample 1 for the same thickness. It is almost the highest reflection absorption property ever reported for such composites.28,43 When the mass ratio increases further, for sample 3 and sample 4, the minimum values of RL are −45.3 dB at 13.5 GHz (d = 2 mm) and −40 dB at 11 GHz (d = 2.5 mm), respectively, and the ranges of RL < −10 dB widen with decreasing Fe3O4 nanoparticles loading. It is worth noting that sample 3 (d = 2.5 mm) has a RL value less than −10 dB which covers whole X-band (8–12 GHz) and this value for sample 4 almost covers a majority of Ku-band (12–18 GHz). It indicates that graphene can improve the microwave loss greatly, because of the connection of dielectric loss and magnetic loss. Compared with other works, the results in this work are better.10,16,43–47 For example, Cui et al.10 reported the Fe3O4 decorated on the single-wall carbon nanoborns showing absorption bandwidth with 9.2 GHz (RL ≤ −10 dB), but the max RL value only −38.8 dB. A higher max RL value was obtained with −53.5 dB, but the reflection loss below −10 dB (RL ≤ −10 dB) only 2.8 GHz in the PANI/GO/Fe3O4 composites.47 Moreover the graphene–Fe3O4 nanohybrids43 exhibited that the frequency range was 9.5 GHz (from 5.1 to 14.6 GHz) for the reflection loss below −10 dB, but the maximum reflection loss reaches −40.4 dB at 7 GHz for the absorber with thickness of 5 mm (Table 1). And in our work, the composites exhibit stronger and wider-frequency wave-absorbing properties.
 | ||
| Fig. 9 Reflection loss curves of (a) Fe3O4, (b) graphene, (c) sample 1, (d) sample 2, (e) sample 3, and (f) sample 4 with different thicknesses. | ||
| Sample | Max RL value (dB) | d (mm) (max RL value) | Frequency (GHz) (max RL value) | Frequency range (GHz) | Effective bandwidth | d (mm) (different frequency range) | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4 nanorods | −40 | 4.4 | ≈2.5 | 44 | |||
| Fe3O4 nanoparticles | −40 | 8.0 | ≈1 | 44 | |||
| Hollow Fe3O4 nanoparticles | <−40 | 55 and 21 | 2.5 and 11.4 | 4–8 (RL ≤ −10 dB) | 4 GHz | 30.0 | 45 |
| Fe3O4 decorated single-wall carbon nanoborns | −38.8 | 5.8 | 3.7 | 3.2–12.4 (RL ≤ −10 dB) | 9.2 GHz | 2–6 | 10 |
| Polyaniline/graphene oxide/Fe3O4 | −53.5 | 3.9 | 7.5 | 6.4–9.2 (RL ≤ −10 dB) | 2.8 GHz | 3.9 | 47 |
| Bowl-like Fe3O4 hollow spheres/reduced graphene oxide | −24.0 | 2.0 | 12.9 | 10.8–15.7 (RL ≤ −10 dB) | 4.9 GHz | 2.0 | 16 |
| Reduced graphene oxide/Fe3O4 composite | −44.6 | 3.9 | 6.6 | 12.2–16.5 (RL ≤ −10 dB) | 4.3 GHz | 3.0 | 46 |
| Graphene–Fe3O4 nanohybrids | −40.4 | 5.0 | 7.0 | 5.1–14.6 (RL ≤ −15 dB) | 9.5 GHz | 2–5 | 43 |
| Hollow Fe3O4/graphene composite | −23.7 | 2.5 | 15.3 | 11–17 (RL ≤ −10 dB) | 6 GHz | 2.5 | This work |
| Flower-like Fe3O4/graphene composites | −53.2 | 2.5 | 11.4 | 9–16 (RL ≤ −10 dB) | 7 GHz | 2.5 | This work |
| Solid sphere Fe3O4/graphene composites | −40.0 | 2.5 | 11.0 | 9.12–16.25 (RL ≤ −10 dB) | 7.13 GHz | 2.5 | This work |
From the results of the composites, it exhibits that when Fe3O4 nanoparticles adhere on the graphene layers uniformly, the dielectric constants and electromagnetic parameters can reach an appropriate value which would improve impedance matching and benefit the microwave loss. Besides, from Fig. 9, we can obviously deduce that the microwave absorption properties could be adjusted by changing the mass ratio. Especially, the sample 2 (flower-like structure) with the optimal ratio (MGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MFe3O4 = 5
MFe3O4 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) shows the strongest absorption (−53.2 dB) and a relative wide range (7 GHz) of RL < −10 dB, which is attributed to the natural resonance mechanism and eddy current effect. As previously stated, the morphology of Fe3O4 nanoparticles changed with the increase of mass ratio from the ring-like sphere, to flower-like sphere and finally to solid sphere. These structures have a crucial influence on the microwave absorption properties.16,48 From the Fig. 10, the schematic view indicates the relationship between the morphology and microwave absorption properties. The microwave absorption is mainly attributed to magnetic loss and dielectric loss, and the microwave is depleted as heat at last. In the sample 1 the ring-like Fe3O4 embedded in the graphene sheet, and the nature resonance and eddy current loss of the Fe3O4 lead to the loss of the microwave. And the interfacial polarization between the two phases can lead to an additional dielectric loss, but the microwave is limited in the hollow cavity by reflecting which confined the heat to the interior, and might weaken the absorption property. On the other hand, the sample 2 and sample 3 have an open structure, and the multi-interfaces between the flower-like sphere, graphitic sheets and paraffin matrix contribute to better properties due to the interfacial electric polarization which enhance the loss of the microwave, and the heat dissipates through the graphene sheet. While comparing sample 2 with sample 3 which has both flower-like particles, we can notice that the RL value for sample 2 is stronger than that for sample 3, because appropriate amount of graphene can optimize the electromagnetic parameters and the impedance matching.
1) shows the strongest absorption (−53.2 dB) and a relative wide range (7 GHz) of RL < −10 dB, which is attributed to the natural resonance mechanism and eddy current effect. As previously stated, the morphology of Fe3O4 nanoparticles changed with the increase of mass ratio from the ring-like sphere, to flower-like sphere and finally to solid sphere. These structures have a crucial influence on the microwave absorption properties.16,48 From the Fig. 10, the schematic view indicates the relationship between the morphology and microwave absorption properties. The microwave absorption is mainly attributed to magnetic loss and dielectric loss, and the microwave is depleted as heat at last. In the sample 1 the ring-like Fe3O4 embedded in the graphene sheet, and the nature resonance and eddy current loss of the Fe3O4 lead to the loss of the microwave. And the interfacial polarization between the two phases can lead to an additional dielectric loss, but the microwave is limited in the hollow cavity by reflecting which confined the heat to the interior, and might weaken the absorption property. On the other hand, the sample 2 and sample 3 have an open structure, and the multi-interfaces between the flower-like sphere, graphitic sheets and paraffin matrix contribute to better properties due to the interfacial electric polarization which enhance the loss of the microwave, and the heat dissipates through the graphene sheet. While comparing sample 2 with sample 3 which has both flower-like particles, we can notice that the RL value for sample 2 is stronger than that for sample 3, because appropriate amount of graphene can optimize the electromagnetic parameters and the impedance matching.
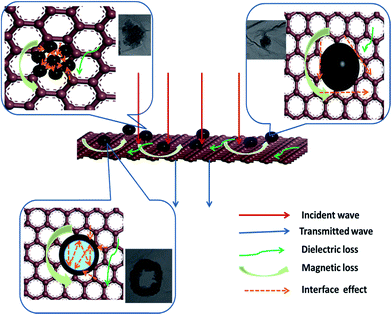 | ||
| Fig. 10 Schematic view of the physical model for the composites with different morphology of Fe3O4 nanoparticles. | ||
Conclusions
In this article, we reported a facile strategy to fabricate the Fe3O4/graphene composites with fantastic electromagnetic wave absorption properties. The structure of Fe3O4 nanoparticles is easily controlled from ring-like sphere, flower-like sphere to solid sphere with increasing mass ratio of GO and Fe3+. The RL peaks of all the composites moves to low frequency with increasing thickness. And the minimum RL value of the different mass ratio of Fe3O4/graphene composites are −25 dB at 17 GHz for sample 1, −53.2 dB at 11 GHz for sample 2, −45 dB at 13.5 GHz for sample 3, and −40 dB at 11 GHz for sample 4, respectively, which are much stronger than pure Fe3O4 nanoparticles. Furthermore, the optimal EMA material was obtained when the Fe3O4 nanoparticles are flower-like structure by controlling the mass ratio of the GO and Fe3+ (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which makes the composite as excellent electromagnetic absorption materials at 2–18 GHz.
1), which makes the composite as excellent electromagnetic absorption materials at 2–18 GHz.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant number 51021002, 51172050, 51102063, 51372052), Natural Scientific Research Innovation Foundation in Harbin Institute of Technology (HIT. NSRIF. 2011109, HIT. NSRIF. 2010121, MH20120757) and the Fundamental Research Funds for the Central Universities (HIT. ICRST. 2010009).References
- F. S. Wen, H. Hou, J. Y. Xiang, X. Y. Zhang, Z. Y. Su, S. J. Yuan and Z. Y. Liu, Carbon, 2015, 89, 372–377 CrossRef CAS.
- D. Micheli, A. Vricella, R. Pastore and M. Marchetti, Carbon, 2014, 77, 756–774 CrossRef CAS.
- L. Kong, X. W. Yin, X. Y. Yuan, Y. J. Zhang, X. M. Liu, L. F. Cheng and L. T. Zhang, Carbon, 2014, 73, 185–193 CrossRef CAS.
- R. C. Che, L. M. Peng, X. F. Duan, Q. Chen and X. L. Liang, Adv Mater, 2004, 16, 401–405 CrossRef CAS.
- M.-S. Cao, J. Yang, W.-L. Song, De-Q. Zhang, B. Wen, H.-B. Jin, Z.-L. Hou and J. Yuan, ACS Appl. Mater. Interfaces, 2012, 4, 6949–6956 CAS.
- R. S. Alam, M. Moradi, M. Rostami, H. Nikmanesh, R. Moayedi and Y. Bai, J. Magn. Magn. Mater., 2015, 381, 1–9 CrossRef CAS.
- Z. M. Cui, L. Y. Jiang, W. G. Song and Y. G. Guo, Chem. Mater., 2009, 21, 1162–1166 CrossRef CAS.
- J. Su, M. H. Cao, L. Ren and C. W. Hu, J. Mater. Chem. C, 2011, 115, 14469–14477 CAS.
- L. Cui, Y. Liu, X. Wu, Z. Hu, Z. Shi, H. Li and A. Govindaraj, RSC Adv., 2015, 5, 75817–75822 RSC.
- L. Cui, Y. Liu, X. Wu, Z. Hu, Z. Shi and H. Li, RSC Adv, 2015, 5, 75817–75822 RSC.
- J. Zheng, H. Lv, X. Lin, G. Ji, X. Li and Y. Du, J. Alloys Compd., 2014, 589, 174–181 CrossRef CAS.
- M. Zong, Y. Huang, Y. Zhao, L. Wang, P. Liu, Y. Wang and Q. Wang, Mater. Lett., 2013, 106, 22–25 CrossRef CAS.
- C. L. Zhu, M. L. Zhang, Y. Qia, G. Xiao, F. Zhang and Y. J. Chen, J. Phys. Chem. C, 2010, 114, 16229–16235 CAS.
- P. B. Liu, Y. Huang and X. Zhang, J. Alloys Compd., 2014, 617, 511–517 CrossRef CAS.
- J. P. Zou, Z. Z. Wang, M. Q. Yan and H. Bi, J. Phys. D: Appl. Phys., 2014, 47, 275001 CrossRef.
- H. L. Xu, H. Bi and R. B. Yang, J. Appl. Phys., 2012, 111, 07A522 Search PubMed.
- Y. J. Zhang, S. Wing and Z. D. Zhang, J. Nanosci. Nanotechnol., 2014, 14, 4664–4669 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- V. K. Singh, A. Shukla, M. K. Patra, L. Saini, R. K. Jani, S. R. Vadera and N. Kumar, Carbon, 2012, 50, 2202–2208 CrossRef CAS.
- T. T. Chen, F. Deng, J. Zhu, C. F. Chen, G. B. Sun, S. L. Ma and X. J. Yang, J. Mater. Chem., 2012, 22, 15190–15197 RSC.
- G. Wang, Z. Gao, G. Wan, S. Lin, P. Yang and Y. Qin, Nano Res., 2014, 7, 704–716 CrossRef CAS.
- P. B. Liu, Y. Huang, L. Wang, M. Zong and W. Zhang, Mater. Lett., 2013, 107, 166–169 CrossRef CAS.
- B. Wen, M. Cao, M. Lu, W. Cao, H. Shi, J. Liu, X. Wang, H. Jin, X. Fang, W. Wang and J. Yuan, Adv. Mater., 2014, 26, 3484–3489 CrossRef CAS PubMed.
- W. Liu, C. Li, P. Zhang, L. Tang, Y. Gu, Y. Zhang, J. Zhang, Z. Liu, G. Sun and Z. Zhang, RSC Adv., 2015, 5, 73993–74002 RSC.
- C. Basavaraja, W. J. Kim, Y. D. Kim and D. S. Huh, Mater. Lett., 2011, 65, 3120–3123 CrossRef CAS.
- J. Zheng, H. L. Lv, X. H. Lin, G. B. Ji, X. G. Li and Y. W. Du, J. Alloys Compd., 2014, 589, 174–181 CrossRef CAS.
- M. Zong, Y. Huang, Y. Zhao, X. Sun, C. H. Qu, D. D. Luo and J. B. Zheng, RSC Adv., 2013, 3, 23638–23648 RSC.
- X. H. Li, H. B. Yi, J. W. Zhang, J. Feng, F. S. Li, D. S. Xue, H. L. Zhang, Y. Peng and N. J. Mellors, J. Nanopart. Res., 2013, 15, 1472 CrossRef.
- C. Hu, Z. Mou, G. Lu, N. Chen, Z. Dong, M. Hua and L. Qu, Phys. Chem. Chem. Phys., 2013, 15, 13038–13043 RSC.
- Y. Wen, Y. J. Zhu, A. Langrock, A. Manivannan, S. H. Ehrman and C. S. Wang, Small, 2013, 9, 2810–2816 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- D. C. Wei, Y. Q. Liu, Y. Wang, H. L. Zhang, L. Q. Huang and G. Yu, Nano Lett., 2009, 9, 1752–1758 CrossRef CAS PubMed.
- E. Ma, J. J. Li, N. Q. Zhao, E. Z. Liu, C. N. He and C. S. Shi, Mater. Lett., 2013, 91, 209–212 CrossRef CAS.
- J. Qiu and T. T. Qiu, Carbon, 2015, 81, 20–28 CrossRef CAS.
- K. Singh, A. Ohlan, V. H. Pham, R. Balasubramaniyan, S. Varshney, J. Jang, S. Hyun Hur, W. Mook Choi, M. Kumar, S. K. Dhawan, B.-S. Kong and J. S. Chung, Nanoscale, 2013, 5, 2411–2420 RSC.
- D. F. Zhang, F. X. Xu, J. Lin, Z. D. Yang and M. Zhang, Carbon, 2014, 80, 103–111 CrossRef CAS.
- H. L. Yu, T. S. Wang, B. Wen, M. M. Lu, Z. Xu, C. L. Zhu, Y. J. Chen, X. Y. Xue, C. W. Sun and M. S. Cao, J. Mater. Chem., 2012, 22, 21679–21685 RSC.
- H. Hu, Z. B. Zhao, Q. Zhou, Y. Gogotsi and J. S. Qiu, Carbon, 2012, 50, 3267–3273 CrossRef CAS.
- S. GB, D. BX, C. MH, W. BQ and H. CW, Chem. Mater., 2011, 23, 1587–1593 CrossRef.
- L. Jia, C. Wenqiang, J. HaiBo, Z. Deqing and C. Maosheng, Journal of Materials Chemistry C, 2015, 3, 4670–4677 RSC.
- X. G. Liu, D. Y. Geng, H. Meng, P. J. Shang and Z. D. Zhang, Appl. Phys. Lett., 2008, 92, 173117 CrossRef.
- C. Wang, X. J. Han, P. Xu, X. L. Zhang, Y. C. Du, S. R. Hu, J. Y. Wang and X. H. Wang, Appl. Phys. Lett., 2011, 98, 072906 CrossRef.
- T. Wang, Z. Liu, M. Lu, B. Wen, Q. Ouyang, Y. Chen, C. Zhu, P. Gao, C. Li, M. Cao and L. Qi, J. Appl. Phys., 2013, 113, 024314 CrossRef.
- M. Jazirehpour and S. A. S. Ebrahimi, J. Alloys Compd., 2015, 638, 188–196 CrossRef CAS.
- Z. W. Li and Z. H. Yang, J. Magn. Magn. Mater., 2015, 387, 131–138 CrossRef CAS.
- M. Zong, Y. Huang, Y. Zhao, L. Wang, P. Liu, Y. Wang and Q. Wang, Mater. Lett., 2013, 106, 22–25 CrossRef CAS.
- J. Zhao, J. Lin, J. Xiao and H. Fan, RSC Adv, 2015, 5, 19345–19352 RSC.
- R. F. Zhuo, H. T. Feng, J. T. Chen, D. Yan, J. J. Feng, H. D. J. Li, B. S. Geng, S. Cheng, X. Y. Xu and P. X. Yan, J. Phys. Chem. C, 2008, 112, 11767–11775 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns of Fe3O4 nanoparticles (Fig. S1), TEM images of nanoparticles of graphene and Fe3O4 (Fig. S2–5), and SEM and TEM images of sample 4 (Fig. S6), SEM images of sample 1 with different reaction time (Fig. S7). See DOI: 10.1039/c5ra22254k |
| This journal is © The Royal Society of Chemistry 2016 |