Facile fabrication of tea tree oil-loaded antibacterial microcapsules by complex coacervation of sodium alginate/quaternary ammonium salt of chitosan†
Abstract
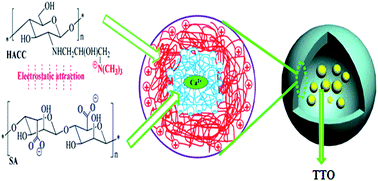
In this study, flavour tea tree oil (TTO)-loaded antibacterial microcapsules were developed based on the complex coacervation of sodium alginate (SA) and a quaternary ammonium salt of chitosan (HACC). The optimum preparation conditions of the TTO-loaded microcapsules determined using the response surface methodology (RSM) were as follows: a weight ratio of the core to wall material (core-wall ratio) of 1 : 1, a pH value of 6.0 and a mass concentration of CaCl2 solution of 0.6 w/v%, at which the resultant microcapsules showed the greatest actual encapsulation efficiency (EE) of 66.06% ± 2.53%. Thereafter, the resultant microcapsules were characterized in terms of morphology, size, components and thermal stability using scanning electron microscopy (SEM), a laser particle diameter analyzer (LPDA), Fourier transform infrared spectroscopy (FTIR), and thermal gravity-differential thermal analysis (TG-DTA) and differential scanning calorimetry (DSC), respectively. Furthermore, both the in vitro drug release and antimicrobial properties of the microcapsules were also assessed. The results displayed that the TTO-loaded microcapsules had a spherical shape with the particle sizes in the range from 1.91 μm to 13.18 μm. The microcapsules possessed outstanding performances with respect to the thermal stability, sustained release activity, antimicrobial effect and long-term inhibition activity. The release profiles of TTO from the microcapsules could be well described by the Ritger–Peppas model. Based on the above research results, the simple and environmentally-friendly microencapsulation formulation could effectively improve the stability and prolong the antimicrobial efficacy of TTO. The microcapsules may find applications in food, cosmetics and medicine.

 Please wait while we load your content...
Please wait while we load your content...