Facile synthesis of novel albumin-functionalized flower-like MoS2 nanoparticles for in vitro chemo-photothermal synergistic therapy†
Liang Chena,
Wei Fenga,
Xiaojun Zhoub,
Kexin Qiub,
Yingke Miaoa,
Qianqian Zhanga,
Ming Qina,
Lei Li*c,
Yanzhong Zhanga and
Chuanglong He *ab
*ab
aCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China. E-mail: hcl@dhu.edu.cn; Fax: +86 21 6779 2742; Tel: +86 21 6779 2742
bState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai 201620, China
cDepartment of Gastroenterology, Shanghai Tianyou Hospital, Tongji University, Shanghai 201620, China. E-mail: lilei2968900@163.com
First published on 25th January 2016
Abstract
Synergistic therapy, the combination of photothermal therapy and chemotherapy, has been becoming an attractive strategy to circumvent certain drawbacks in current cancer chemotherapy. Herein, a synergistic therapeutic nanoplatform based on novel albumin functionalized flower-like MoS2 spherical nanoparticles was reported. The MoS2 nanoparticles were prepared by using hydrazine hydrate as both reductive agent and structure-directing agent via a facile hydrothermal procedure. Bovine serum albumin (BSA) was subsequently modified onto the surface of MoS2 (MoS2@BSA) to improve the physiological stability and biocompatibility of nanoparticles. The results indicated that the prepared MoS2@BSA hybrid nanoparticles not only possess high photothermal conversion efficiency and physiological stability, but also the ability to effectively load and intelligently release the chemotherapeutic drug doxorubicin hydrochloride (DOX). The in vitro cytotoxicity of MoS2@BSA was tested with murine breast cancer cells (4T1), revealing the excellent biocompatibility of hybrid nanoparticles. Furthermore, the negligible hemolytic activity and efficient cellular uptake of MoS2@BSA was also testified. More importantly, the combination of DOX loading and photothermal treatment with MoS2@BSA in the form of MoS2@BSA–DOX displayed better therapeutic efficacy than single photothermal therapy or chemotherapy. Thus, our results demonstrated that the novel combined therapeutic nanoplatform based on the flower-like MoS2 hybrid nanoparticles could be an attractive candidate for cancer treatments.
Introduction
Over the past decades, chemotherapy has always been considered as one of the most commonly used approaches for cancer treatment. Unfortunately, the available chemotherapeutic drugs involve some inevitable shortcomings such as severe side effects, low efficiency and drug resistance. To overcome these limitations, various strategies including targeted drug delivery system,1,2 enhanced cell uptake of drugs3 and combination of different therapeutic approaches,4 have been intensively studied to improve the anticancer efficacy and optimize therapy. Benefiting from its minimally invasive feature and fewer side effects, photothermal therapy has emerged as a new promising alternative for cancer treatment. Upon exposure to near-infrared (NIR) light, the heat energy converted by photothermal agents could not only be applied to kill cancer cells in situ by hyperthermia, but is also able to greatly enhance the efficacy of chemotherapeutics drug under mild heating.5 Therefore, the integration of photothermal therapy with chemotherapy may be an attractive strategy to circumvent the certain drawbacks in current cancer chemotherapy, owing to its prominent synergistic effect.6Up to now, a variety of photothermal agents have shown encouraging thermal therapeutic efficacy in vitro and in vivo experiments.7 For example, semiconductor nanomaterials (CuS,8 Cu2−xSe,9 Cu9S5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), Nd3+-doped LaF3 nanocrystals11 and organic conjugated polymer12,13 had been demonstrated for effective photothermal therapy. Besides, gold nanostructures with various sizes and morphologies (such as gold nanorods,14 gold nanocage,15 gold nanoshell,4 gold nanostar16,17) are the most widely studied photothermal agents because of the efficient conversion of optical energy into heat based on surface plasmon resonance (LSPR) absorption. However, some intrinsic drawbacks including high cost and the low photostability severely restrict their clinical applications.16 Carbon nanomaterials, including carbon nanotubes,18 carbon nanohorns19,20 and graphene,21 are another class of extensively studied photothermal agents, while the relatively low absorption coefficients in NIR region of carbon nanomaterials has also been debated.22 From this viewpoint, the developments of novel photothermal agents with strong NIR absorbance as well as excellent photostability are highly desirable.
10), Nd3+-doped LaF3 nanocrystals11 and organic conjugated polymer12,13 had been demonstrated for effective photothermal therapy. Besides, gold nanostructures with various sizes and morphologies (such as gold nanorods,14 gold nanocage,15 gold nanoshell,4 gold nanostar16,17) are the most widely studied photothermal agents because of the efficient conversion of optical energy into heat based on surface plasmon resonance (LSPR) absorption. However, some intrinsic drawbacks including high cost and the low photostability severely restrict their clinical applications.16 Carbon nanomaterials, including carbon nanotubes,18 carbon nanohorns19,20 and graphene,21 are another class of extensively studied photothermal agents, while the relatively low absorption coefficients in NIR region of carbon nanomaterials has also been debated.22 From this viewpoint, the developments of novel photothermal agents with strong NIR absorbance as well as excellent photostability are highly desirable.
Recently, molybdenum disulfide (MoS2), a typical two-dimensional transition metal dichalcogenides possesses similar optical and electronic properties with graphene, have drawn widespread research interest in wide range of fields, including nanoelectronics, sensor and catalysis.23–26 Nevertheless, the exploration of MoS2 in area of biomedicine is still in its infancy. Chou et al. firstly found that the chemical exfoliated MoS2 nanosheets exhibited better photothermal performance than gold nanorods and graphene, which strongly suggested that MoS2 could be used as an efficient photothermal agent for cancer therapy.27 Then the exfoliated MoS2 nanosheets have also been fabricated as drug carrier for chemo-photothermal synergistic therapy.28,29 Besides, the magnetic nanoparticles decorated MoS2 nanosheets was prepared by Liu et al. for multifunctional imaging-guided photothermal therapy.30 Even though these MoS2-based nanocomposites showed effective therapeutic efficacy, the size and morphology of MoS2 nanosheets produced by exfoliated methods are always uncontrollable. More recently, Shi et al. proposed a one-pot solvothermal procedure to synthesis PEGylated MoS2 nanosheets using PEG-400 aqueous solution as the solvent.31 Using similar solvothermal method to fabricate magnetic MoS2 hybrid nanoflakes for theranostics was also reported.32 This solvothermal method could endow the obtained nanomaterials with inherent colloidal stability in physiological environment. However, it may hinder the further functionalization of MoS2 considering the difficulty of ligand incorporation of PEG.33,34 In addition, the morphology of above MoS2 nanosheets was non-uniform, which might also be an obstacle for practical application. Consequently, it's truly meaningful to design facile synthetic route to prepare novel MoS2-based photothermal agent with controllable morphology and easily modified surface for further biomedical applications.
In the previous study, flower-like MoS2 nanoparticles were developed as a new photothermal agent for cancer therapy.35 To further explore the properties of this novel MoS2 nanomaterials, herein, the bioactive molecules bovine serum albumin (BSA), possessing low immunogenicity, outstanding biocompatibility and the presence of abundant functional groups,36 was employed to modify the as-prepared MoS2 (MoS2@BSA). Actually, the BSA had been effectively used to exfoliate MoS2 to form single-layer MoS2 nanosheets with improved biocompatibility.37 Unfortunately, the sonication process was time-consuming (48 h) and no further properties of hybrid nanosheets were investigated. In contrast, this MoS2@BSA hybrid nanoparticles could not only efficiently transduce NIR light to heat for hyperthermia, but can also be utilized as nanocarriers to load doxorubicin hydrochloride (DOX). In addition to its intrinsic photothermal therapeutic effect, photothermal-triggered release of a loaded drug is an advantageous way to obtain synergistic chemo and photothermal effects. The good stability, photothermal effects and biocompatibility of MoS2@BSA were systematically investigated. Then the loading and releasing profiles of DOX from the nanocomposites were evaluated. Moreover, the effective cell uptake and therapeutic efficacy of DOX-loaded hybrid nanoparticles (MoS2@BSA–DOX) were also demonstrated by in vitro studies.
Experimental
Materials
Ammonium tetrathiomolybdate ((NH4)2MoS4) and hydrazine hydrate (N2H4·H2O, 98%) were purchased from J&K Scientific Ltd (Beijing, China). Bovine serum albumin (BSA) was obtained from Sigma-Aldrich (Shanghai) Trading Co., Ltd (Shanghai, China). Doxorubicin hydrochloride (DOX) was provided by Beijing Huafeng United Technology Co., Ltd. Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), trypsin, penicillin (100 U mL−1) and streptomycin (100 μg mL−1) were all purchased from Shanghai Yuan xiang medical equipment Co. Cell Counting Kit-8 (CCK-8) and propidium iodide (PI) were obtained from Beyotime Institute of Biotechnology (China). Acridine orange (AO) was obtained from Shanghai BestBio Co. All other reagents were used without any further purification. Deionized (DI) water (18.2 MΩ cm resistivity) was used for all experiments.Preparation of flower-like MoS2 nanoparticles
The bare flower-like MoS2 nanoparticles were prepared by hydrothermal route according to previous report.35,38 Briefly, 40 mg of (NH4)2MoS4 was fully dissolved in 18 mL DI water with the aid of ultrasonic bath. Then 0.18 mL of N2H4·H2O was added and the bright orange-red solution was vigorous stirring at room temperature for 30 min. The mixture was then transferred into a 40 mL Teflon-lined stainless steel autoclave and maintained at 200 °C for 10 h. Finally, the black products were collected by centrifugation and washed with DI water for several times to obtain pure MoS2 nanoparticles.Preparation of MoS2@BSA
To improve the physiologic stability of MoS2 nanoparticles, the MoS2 nanoparticles were modified with BSA. Typically, 200 mg of BSA was added into 20 mL aqueous solution of MoS2 nanoparticles (1 mg mL−1). After stirring for 3 h at room temperature, the excess BSA was removed by repeated centrifugation and the as-prepared MoS2@BSA hybrid nanoparticles were re-dispersed in DI water and stored at 4 °C for further use.Characterization
The morphology and structure of the nanoparticles were characterized by a Hitachi S-4800 (Hitachi Ltd., Japan) field emission scanning electron microscope (FESEM) at 10 kV and a JEM-2100F (JEOL Ltd., Japan) transmission electron microscope operating at 200 kV. The particle size distributions of flower-like nanoparticles were analysed by dynamic light scattering (DLS) using a BI-200SM multiangle dynamic/static laser scattering instrument (Brookhaven, USA). The Raman spectra were performed at room temperature by using an inVia-Reflex micro-Raman spectroscopy system (Renishaw, UK) with a 633 nm solid laser of 50 mW power. Fourier transform infrared spectroscopy (FTIR) spectra were recorded using a Nexus 670 (Thermo Nicolet, USA) spectrometer using KBr pellets. Zeta potential was determined in a Zetasizer Nano ZS apparatus (Malvern, UK). UV-vis-NIR absorption spectra were obtained using a Lambda 35 UV-vis spectrophotometer (PerkinElmer, USA). The Mo concentration was measured using a Leeman Prodigy Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) system (Hudson, NH03051, USA).Photothermal effects of MoS2@BSA nanoparticles
To investigate the photothermal performance of MoS2@BSA nanoparticles, 0.2 mL different concentration MoS2@BSA nanoparticles were irradiated with an 808 nm continuous-wave laser device (SFOLT Co., Ltd, Shanghai, China) for 5 min. The temperature of the solutions was recorded every 30 seconds by using a thermocouple thermometer (DT-8891E, Shenzhen Everbest Machinery Industry Co., Ltd, China) with a thermocouple probe.DOX loading and releasing
For DOX loading, the as-prepared MoS2@BSA were dispersed in PBS (0.5 mg mL−1) and mixed with DOX aqueous solution at concentration of 1 mg mL−1 (volume ratio = 5/1). The mixtures were stirred in dark at room temperature for 24 h. After that, the MoS2@BSA–DOX nanoparticles were collected by centrifugation and repeatedly washed with PBS to remove excess DOX. The supernatant and the washing solutions were collected together and the amount of un-loaded DOX was determined by UV-vis spectra at wavelength of 480 nm. The DOX loading content was calculated by using the following equations:39
 | (1) |
For in vitro DOX releasing, MoS2@BSA–DOX (2 mg) were soaked in 2 mL solution and transferred into a dialysis bag (molecular weight cut-off = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). After then, the dialysis bag was immersed in 10 mL buffer solution with different pH values (pH 5.0 or pH 7.4) and shake slightly at 37 °C. At predetermined time intervals, 10 mL of samples were withdrawn for analysis and equivalent fresh buffer solution were added back. To investigate photothermal-triggered drug release from the MoS2@BSA–DOX nanoparticles, MoS2@BSA–DOX were dispersed in 2 mL of buffer solution at different pH values. At certain time intervals, the solution was irradiated with 808 nm NIR laser for 10 min. 100 μL of samples was taken out before and after NIR irradiation and centrifuged to remove nanoparticles. The supernatant was then diluted by corresponding buffer solution for analysis. The amount of released DOX was all quantified by UV-vis spectrometry.
000). After then, the dialysis bag was immersed in 10 mL buffer solution with different pH values (pH 5.0 or pH 7.4) and shake slightly at 37 °C. At predetermined time intervals, 10 mL of samples were withdrawn for analysis and equivalent fresh buffer solution were added back. To investigate photothermal-triggered drug release from the MoS2@BSA–DOX nanoparticles, MoS2@BSA–DOX were dispersed in 2 mL of buffer solution at different pH values. At certain time intervals, the solution was irradiated with 808 nm NIR laser for 10 min. 100 μL of samples was taken out before and after NIR irradiation and centrifuged to remove nanoparticles. The supernatant was then diluted by corresponding buffer solution for analysis. The amount of released DOX was all quantified by UV-vis spectrometry.
Cell culture
Murine breast cancer cells (4T1) were purchased from Chinese Academy of Sciences Cell bank for Type Culture Collection (Shanghai, China) and were routinely cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin, respectively. The cells were grown in a humidified incubator at 37 °C with 5% CO2.In vitro cytotoxicity of MoS2@BSA nanoparticles
The cytotoxicity of hybrid nanoparticles against 4T1 cells was evaluated by standard CCK-8 assays. Briefly, 4T1 cells were seeded in 96-well plates at density of 1 × 104 cell per well and incubated overnight. Then medium was changed with a fresh medium (negative control) and medium containing different concentrations of MoS2@BSA nanoparticles. After incubated for 24 h, the culture medium was removed and each well was washed twice with PBS. Then 100 μL of serum-free medium containing 10 μL of CCK-8 reagent were added into each well. After incubated for another 2 h, the cell numbers were determined by measuring the absorbance at 450 nm using a microplate reader (Multiskan MK3, Thermo). The relative cell viability was calculated by eqn (2), and the average value was obtained from five parallel experiments.
 | (2) |
The ODtest and ODcontrol are the OD value of cells treated with and without MoS2@BSA nanoparticles, respectively.
To further verify the cytocompatibility of the MoS2@BSA hybrid nanoparticles, the 4T1 cells were seeded in 12-well plates at density of 105 cells per well and incubated for 24 h. After that, the cells were incubated with MoS2@BSA (200 μg mL−1) for 24 h and 48 h. Then the cell medium were discarded and the cells were washed with PBS for three times, the morphology of cell was directly observed and imaged by an inverted microscope with a magnification of 100 for each sample.
In vitro hemolysis assays
Hemolysis assay of MoS2@BSA nanoparticles was carried out according to our previous reports.31,32 Red blood cells (RBCs) were harvested from 1 mL mice blood via centrifugation and washed with PBS for several times. The purified RBCs were diluted by PBS. Then 0.3 mL of diluted RBCs suspension was mixed with 1.2 mL of MoS2@BSA suspensions at desired concentrations. Equal volume of PBS and water were used instead of samples for negative and positive control, respectively. Afterwards, the samples were vortexed and incubated at 37 °C for 3 h, followed by centrifugation at 3000 rpm for 3 min. Finally, the absorbance of the supernatants at 541 nm was measured by using UV-vis spectrophotometer. The hemolysis percent of RBCs was calculated by eqn (3):
 | (3) |
In vitro cellular uptake assay
The cellular uptake of MoS2@BSA nanoparticles was also investigated according to similar protocols in the literature,37,41 in detail, 4T1 cells were seeded in 6-well plates with a density of 2 × 105 cell per well. After incubated for 24 h, the medium was replaced with fresh medium containing MoS2@BSA nanoparticles at concentration of 50 and 100 μg mL−1, followed by 24 h incubation. Then the medium was discarded and the cells were rinsed with PBS for three times, trypsinized and collected by centrifugation. Finally, the obtained samples were treated with aqua regia solution (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 1
HNO3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, volume ratio) for 2 h and diluted with PBS to determine the Mo concentration by ICP-AES.
3, volume ratio) for 2 h and diluted with PBS to determine the Mo concentration by ICP-AES.
Bio-TEM was carried out to further examine the cellular uptake ability of hybrid nanoparticles. Briefly, 4T1 cells were incubated with MoS2@BSA nanoparticles (50 μg mL−1) for 24 h. Thereafter, the cells were washed with PBS, trypsinized and collected by centrifugation. The cells were fixed in PBS solution containing 2.5% glutaraldehyde at 4 °C for 2 h. Then they were washed three times with PBS and fixed in 1% osmium tetroxide solution for 2 h, dehydrated through a graded series of ethanol solution, and embedded in epoxy resin to polymerize in the oven at 37 °C for 12 h, 45 °C for 12 h, and 60 °C for 48 h. Ultrathin sections of approximately 70 nm were cut by ultramicrotome and stained with 5% aqueous uranyl acetate and 2% aqueous lead citrate and then observed by JEM-2100TEM.
Cellular uptake of MoS2@BSA–DOX
To visualize the cellular uptake of MoS2@BSA–DOX, 4T1 cells were seeded in 12-well plates (about 5 × 104 cells per well) and incubated for 24 h. Then medium was taken out and the cells were rinsed with PBS then treated with free DOX and MoS2@BSA–DOX nanoparticles (DOX concentration at 2 μg mL−1 and 5 μg mL−1). After incubation for 2 h, the cells were rinsed with PBS and directly observed by inverted fluorescence microscope.Furthermore, the cellular uptake capability of nanoparticles was quantitatively measured by flow cytometry (FCM). 4T1 cells were incubated with free DOX and MoS2@BSA–DOX nanoparticles at DOX concentration of 2 and 5 μg mL−1 for 2 h. Then the cells were rinsed with PBS, trypsinized and collected by centrifugation. The obtained cells were washed with PBS twice, suspended in 1 mL of PBS and filtered through 400-mesh sieves. Finally, the cell suspensions were subjected to FACS Calibur flow cytometry (BectonDickinson, USA) equipped with an argon laser (488 nm) and analyzed with Cell Quest software through the fluorescence channel 2 (FL2) for DOX.
In vitro chemo-photothermal therapeutic effects
To evaluate the synergistic therapy effects of MoS2@BSA–DOX nanoparticles, 4T1 cells were cultured in 96-well plates with 0.1 mL of medium at density of 104 cells per well. After washed twice with PBS, the cells were incubated with medium containing free DOX, MoS2@BSA and MoS2@BSA–DOX at predetermined DOX concentration (20 μg mL−1) for 3 h, while groups of MoS2@BSA, MoS2@BSA–DOX have an equivalent MoS2@BSA dosage (∼58 μg mL−1). For groups of photothermal and photothermal chemotherapy, the cells were exposed to 808 nm NIR laser (1 W cm−2) for 10 min. Then the cells were rinsed with PBS for three times and cultured for another 21 h. The relative cell viability was measured by CCK-8 assay in the same procedure as described in above. All experiments were carried out in triplicate.Furthermore, the chemo-photothermal therapeutic effects were also intuitively verified by AO/PI double staining assay. Briefly, 4T1 cells were incubated in a 24-well culture plate at a density of 2 × 104 cells per well. Afterwards, the cells were treated with free DOX, MoS2@BSA or MoS2@BSA–DOX for 3 h. Then the corresponding groups were irradiated by 808 nm NIR laser (1 W cm−2) for 10 min. After that, the cells was washed with PBS and cultured for another 12 h. Finally, the cells were stained with AO–PI for 30 min and immediately observed and imaged by inverted fluorescence microscope.
Statistical analysis
The data were presented as the mean ± standard deviation (SD). The statistical analysis was carried out by using one-way analysis of variance (one-way ANOVA) and Scheffe's post hoc test. The statistical significance for all tests was considered at *P < 0.05 and **P < 0.01.Results and discussion
Synthesis and characterization of MoS2@BSA nanoparticles
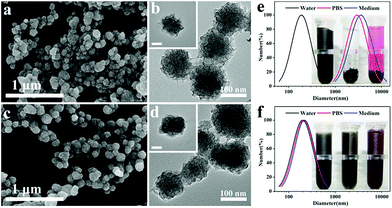
The synthetic procedure of the combined therapeutic nanoplatform was illustrated in Scheme 1. For synthesis of flower-like MoS2 nanoparticles, we employed a facile hydrothermal method to reduce (NH4)2MoS4 to MoS2 with hydrazine hydrate. Compared with the traditional up-down strategy to prepare MoS2 nanosheets by chemical exfoliation method, the hydrothermal route is more convenient and reproducible. The morphology of the nanoparticles was investigated by FESEM and TEM. The as-prepared particles have almost spherical profile and highly rough surface, which consists of many random protrusions (Fig. 1a). The TEM images could further confirm the formation of spherical particles with relatively uniform size of around 100 nm (Fig. 1b). Meanwhile, it clearly showed that the flower-like structure was built from abundant irregular and thin curving nanosheets. It's interesting to note that MoS2 prepared without hydrazine hydrate has no flower-like shape but large agglomerates composing of numerous disordered nanosheets (Fig. S1†).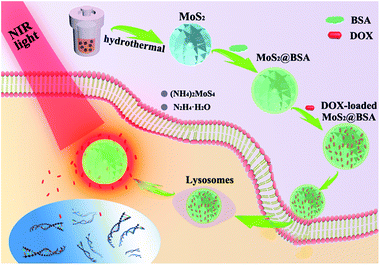 | ||
| Scheme 1 Schematic illustration of albumin functionalized flower-like MoS2 as a novel nanoplatform for chemo-photothermal synergistic therapy. | ||
Prior to formulate the obtained MoS2 nanoparticles for biomedical applications, bioactive molecules BSA was assembled onto the surface of flower-like MoS2 nanoparticles via physical absorption to enhance its biocompatibility and physiologic stability.42 As shown in Fig. 1c, the surface of hybrid nanoparticles became slightly smooth after coating with BSA. The thick organic shell was apparently visible from TEM images (Fig. 1d). The DLS measurement was also carried out to verify the successful modification of BSA and the physiologic stability of hybrid nanoparticles. The hydrodynamic size of nanoparticles suspended in water was increased from 188.5 nm to 198.8 nm after coating with BSA. Moreover, the size of nanoparticles dispersed in PBS and cell medium were tested as well by DLS. For the bare flower-like MoS2, the nanoparticles inevitably aggregate and precipitate in PBS and cell medium because of the electron screen effect,28 which results in an significant increase of hydrodynamic size (Fig. 1e). However, the dispersions of hybrid nanoparticles were stable without any precipitation and the hydrodynamic size of MoS2@BSA were almost unchanged in both PBS and cell medium, which demonstrated that the modification of BSA would effectively ensure the physiologic stability of nanoparticles (Fig. 1f).
Furthermore, other physicochemical properties of nanoparticles were also studied. Fig. 2a shows the Raman spectra of MoS2 and MoS2@BSA hybrid nanoparticles. The typical in-plane E12g peak located around 377 cm−1 and out-of-plane A1g peak located at 403 cm−1 were identically emerged on the spectra of MoS2 and MoS2@BSA. The red shifting and broadening of Raman bands were similar with previously reported fullerene-like MoS2 nanoparticles43 and multiwall MoS2 nanotubes,44 suggesting that layers structure present in these samples45 and the lateral dimensions of these layers are in the nanoregime.46 The existence of BSA on flower-like MoS2 was also elucidated by FTIR spectra. In Fig. 2b, the MoS2@BSA displayed two characteristic absorption bands around 1647 and 1524 cm−1 compared to MoS2, which were assigned to the amide I and amide II bands of BSA, respectively.47 The peaks at about 2923 and 2851 cm−1 were contributed to the C–H stretching on the skeleton of BSA. Afterwards, zeta potential measurement was performed to evaluate the surface charge of nanoparticles suspended in water, PBS and medium (Fig. 2c). The strongly negative surface charge of the flower-like nanoparticles (−45.3 mV) assured its excellent colloidal stability in aqueous media. The zeta potential of hybrid nanoparticles decreased slightly after modification of BSA. Notably, the zeta potential of both MoS2 and MoS2@BSA in PBS and medium were all reduced as compared with their aqueous dispersion, which may result from compression of the electrical double layer with the increased ionic strength.48 The UV-vis spectra of MoS2 and MoS2@BSA were shown in Fig. 2d. Since they have equally strong absorption in UV to visible region, it was readily concluded that the coating of BSA didn't affect the optical absorption of MoS2.
Photothermal effects of MoS2@BSA nanoparticles
We next evaluated the photothermal properties of MoS2@BSA nanoparticles to verify the hyperthermia capacity. The temperature variation of MoS2@BSA aqueous solution with different concentrations irradiated by an 808 nm laser was recorded. The ID water was used as negative control. As shown in Fig. 3a, the photothermal heating curves of MoS2@BSA displayed obvious concentration-dependent effects. For instance, the temperature of MoS2@BSA solution at concentration of 200 μg mL−1 elevated rapidly from 23.7 to 72.4 °C after 5 min irradiation. In contrast, the temperature of pure water increased only 0.8 °C under same conditions. Meanwhile, the temperature of MoS2@BSA solution (50 μg mL−1) rose higher with the increase of NIR laser power density (Fig. 3b).Another prerequisite of effective photothermal therapy is the high photostability of photothermal agent. Therefore, we monitored the temperature of MoS2@BSA aqueous solution under on–off cycles of the NIR irradiation. As depicted in Fig. 3c, the MoS2@BSA remained its initial excellent photothermal effect without any weakening of temperature elevation. Additionally, the hybrid nanoparticles presented almost the same absorbance in NIR regions after exposed to NIR irradiation for even five cycles (Fig. 3d). The above results forcefully certified that the MoS2@BSA could efficiently convert NIR laser energy into heat energy and possess remarkable photostability. Thus, the as-prepared flower-like MoS2 hybrid nanoparticles are highly profitable for NIR laser-driven photothermal therapy.
DOX loading and releasing behavior
Apart from the outstanding photothermal transduction ability, the 2D nature of MoS2 and the high surface area of flower-like structure are also expected to load small molecular drugs for chemotherapy. To evaluate the drug loading and releasing properties of MoS2@BSA nanocarriers, DOX was chosen as model drug and loaded into the MoS2@BSA. The UV-vis spectra were utilized to testify the successful loading of DOX. As shown in Fig. 4a, the intensity of characteristic absorption peaks of DOX in initial solution observably decreased after mixed with MoS2@BSA hybrid nanoparticles and it accordingly appeared in the UV-vis spectrum of MoS2@BSA–DOX solution, declaring that most DOX molecules were directly clustered into the flower-like cavities. Certainly, some of free DOX also might be loaded into the thin BSA shell on surface of flower-like MoS2, considering the electrostatic absorption between them.49 The drug entrapment efficiency and loading content was 85% and 34%, respectively. It is worth noting that the MoS2@BSA–DOX could disperse well in PBS without any agglomerates (inset pictures), suggesting the good stability of the MoS2@BSA–DOX nanocomposite.Moreover, the releasing profile of DOX under different conditions was systematically studied. By dialyzing MoS2@BSA–DOX in phosphate buffer solutions at pH 5.0 and 7.4, the released DOX from the carrier was collected and analyzed by UV-vis spectroscopy. Fig. 4b reveals that the release of DOX from the MoS2@BSA–DOX was pH-dependent and the release rate increased with the decrease of pH value. At pH 7.4, only 2 ± 0.3% of DOX was released within first 48 h. Inversely, much faster release was observed when pH decreased to 5.0, and eventually the release amount of DOX reached to 58.8 ± 1.2% over 48 h. These results were probably attributed to the electrostatic interaction between DOX and BSA. According to previous reports, BSA has many free carboxyl groups, which could bind positively charged amino groups of DOX via electrostatic attractions at pH 7.4,50 thus preventing the leakage of DOX. With the decrease of pH value, the electrostatic interactions between DOX and BSA became weak due to neutral charge of BSA around its isoelectric point (∼5.1), which resulted in easily release of DOX from carrier in acidic environments.51 Therefore, the hybrid MoS2@BSA nanoparticles hold great potential for pH-responsive drug delivery, which would be beneficial for enhanced anti-tumor efficiency because both the intracellular lysosomes and extracellular environment of the tumor are acidic comparing with normal physiological condition.52
To test whether the local heating of photothermal nanocarriers could further promote DOX release from nanocomposite, MoS2@BSA–DOX in different pH buffer solutions were illuminated by 808 nm laser for 10 min at each time intervals. The supernatant solutions before and after laser irradiation was collected by centrifugation and measured by UV-vis spectroscopy. The data showed that the accelerated release of DOX was realized after NIR irradiation (Fig. 4c). Surely, the NIR light-triggered release process was also pH-dependent. As we seen, the total amount of released DOX under NIR irradiation was higher than that without irradiation at pH 5.0, while rather limited DOX release was noticed at pH 7.4 after exposure to NIR laser. Undoubtedly, the pH-responsive and NIR laser-triggered release of MoS2@BSA–DOX were greatly favorable for chemo-photothermal synergistic therapy.
In vitro cytotoxicity of MoS2@BSA nanoparticles
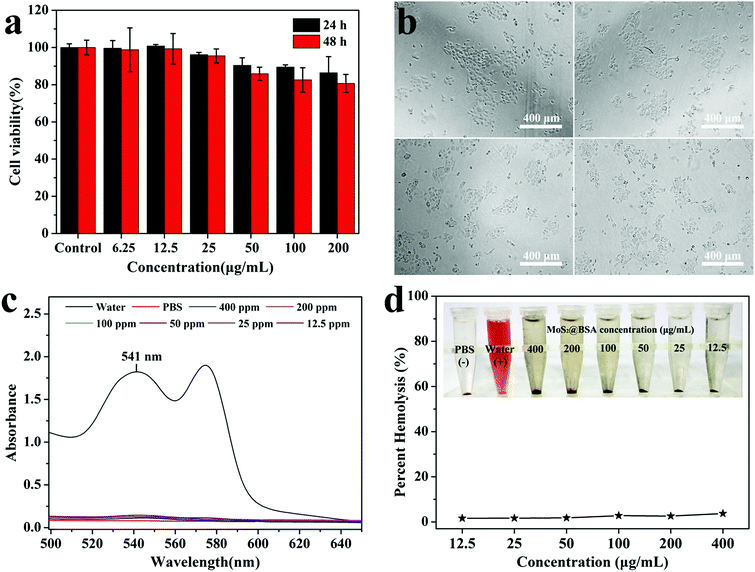
Biocompatibility is an essential concern when we develop the nanoparticles for biomedical applications. In this study, the effect of nanoparticles interaction on viability of 4T1 cells was measured after treatment of different concentrations of MoS2@BSA for 24 h or 48 h by CCK-8 assay. Apparently, the BSA-modified MoS2 exhibited limited toxicity at our tested concentrations (Fig. 5a). The viability of the cells were all greater than 80% after treated with MoS2@BSA for 24 h or 48 h, even at the concentration of as high as 200 μg mL−1. Correspondingly, the cell morphology of 4T1 after 24 and 48 h treatment of MoS2@BSA was observed by inverted microscopy to qualitatively affirm their cytotoxicity. It was clearly shown in the photograph that the cells exhibited no appreciable change in morphology after treatment of MoS2@BSA, which was similar with the control group treated with cell medium (Fig. 5b). The results indicated that the MoS2@BSA had low cytotoxicity, and thus may be used as biocompatible photothermal agents for cancer therapy.Hemocompatibility of MoS2@BSA nanoparticles
Hemocompatibility is of great importance for nanomaterials required to contact with blood. So the in vitro hemolysis assay was performed to investigate the impacts of MoS2@BSA hybrid nanoparticles on the hemolysis behavior of RBCs. Fig. 5c shows the UV-vis spectra of the supernatant of each sample. The hemolysis percentages of MoS2@BSA at different concentrations were quantified based on the absorbance of the supernatant at 541 nm. Negligible hemolysis phenomenon is detected under our studied concentration, similar with the negative PBS control. It was found that even at a high concentration of 400 μg mL−1, the hemolysis percentage of hybrid nanoparticles was less than 4% (Fig. 5d), suggesting the excellent hemocompatibility of MoS2@BSA.Cellular uptake of nanoparticles
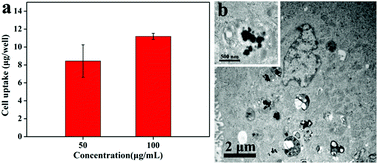
The efficient photothermal therapy requires that the photothermal agents could be readily internalized by cancer cells. Hence, the uptake of MoS2@BSA by 4T1 cells was firstly analyzed by ICP-AES. As shown in Fig. 6a, the uptake content of Mo was increased with the increasing concentration of MoS2@BSA nanoparticles, indicating that the feasible intracellular uptake of MoS2@BSA nanoparticles. More than the quantitative measurement, the internalization of MoS2@BSA into cells was also convinced by TEM (Fig. 6b). Distinctly, large numbers of nanoparticles were observed in the cytoplasm of 4T1 cell while no nanoparticles were found in the nucleus. It can be seen that the nanoparticles still maintained the spherical shape with harsh edge from the high-magnification image. We deduced that the modification of albumin on the surface of MoS2 may have positive influence on its capacity of entering into cancer cells, as the similar trend of gold nanorods has been reported by Qiu et al.53 The image also showed that many hybrid nanoparticles were located in vesicles in the cytoplasm, which demonstrated that the nanoparticles might be internalized by the cells via receptor-mediated endocytosis pathway.31Cellular uptake of MoS2@BSA–DOX
Having established that MoS2@BSA could be internalized by 4T1 cells, the drug delivery ability of MoS2@BSA was further evaluated by monitoring the cellular uptake behavior of MoS2@BSA–DOX with fluorescence microscopy and flow cytometry, where free DOX at the same DOX concentration was employed as control. As shown in Fig. 7a, the red fluorescence of DOX in 4T1 cells treated with MoS2@BSA–DOX was relatively lower than that treated with free DOX at the equivalent concentration. Since DOX is a small molecule drug, DOX could readily diffuse through the cell membrane and rapidly enter the cell nucleus.40 In contrast, as a nanocarrier, MoS2@BSA–DOX are mainly internalized into the cells via aforementioned receptor-mediated endocytosis and localized in endosomes/lysosomes, which may result in slower cellular uptake of MoS2@BSA–DOX and thereby delayed DOX release from the carriers. Quantitative results from flow cytometry also accorded with the same tendency (Fig. 7b). The mean fluorescence intensity of both free DOX and MoS2@BSA–DOX treated cells were increased with the concentration of DOX. Therefore, we concluded that the MoS2@BSA could efficiently delivery DOX into cancer cells, which is beneficial for chemo-photothermal combined cancer therapy.In vitro combined synergistic therapy
Inspired by its admirable photothermal effect and drug delivery capability, the in vitro synergistic therapeutic efficiency of MoS2@BSA–DOX on 4T1 cells was evaluated in detail. AO and PI double staining assay were firstly conducted to quantitatively measure the cell viability of 4T1 cells after treated with free cell medium, MoS2@BSA and MoS2@BSA–DOX with or without NIR laser irradiation. AO is a weak base dye and able to readily penetrate through the cell membranes of bath normal and dead cells, while PI is only taken by dead cells with damaged membranes and cause them to fluoresce bright red, resulting in bright green and orange fluorescence in the normal and dead cells, respectively.53 As expected, compared with the only chemotherapeutic treatment group (Fig. 8b), high rate of cell death was observed in NIR-irradiated photothermal groups (Fig. 8c and d), which might be caused by the strong heat-induced damage of photothermal effect.54 In sharp contrast, the control groups without any treatment displayed all green colors in image (Fig. 8a).Next, the CCK-8 assay was also performed to quantitatively assess the synergistic therapeutic effect of MoS2@BSA–DOX. As shown in Fig. 8e, the free DOX exhibited higher toxicity than MoS2@BSA–DOX at the same DOX concentration, owing to the faster cell uptake of free DOX mentioned above. Distinctly, the chemo-photothermal synergistic therapy lead to significantly higher cell mortality rate than single photothermal therapy or chemotherapy (P < 0.05), which was consistent with the qualitative results. In addition, the nominal efficiency of additive therapeutic therapy (Tadditive) with the combination of photothermal and chemotherapy could be calculated by eqn (4):55
| Tadditive = (1 − fchemo × fthermal) × 100% | (4) |
Conclusions
In summary, a synergistic chemo-photothermal therapeutic nanoplatform based on BSA coated flower-like MoS2 nanoparticles was successfully fabricated and well characterized. The functionalization of BSA could endow the flower-like MoS2 with outstanding physiological stability, hemocompatibility and lower cytotoxicity. The inner flower-like MoS2 can not only absorb and convert NIR laser into heat with high photostability, but also be utilized as nanocarrier to effectively loading chemotherapeutic drug DOX with pH- and NIR-responsive releasing behavior. Furthermore, our results suggested that the multifunctional nanoplatform could be uptake by 4T1 cells and eventually delivery DOX into cancer cells. An effective treatment of 4T1 cells in vitro under NIR irradiation was demonstrated, suggesting that synergistic therapeutic efficacy of photothermal and chemotherapy was better than photothermal or chemotherapy alone. As all these desirable characteristics were concentrated in the easily fabricated nanoplatform, we anticipate that the flower-like hybrid nanoparticles will turn out to be a promising cancer therapeutic agent.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (31271028, 31570984), Innovation Program of Shanghai Municipal Education Commission (13ZZ051), International Cooperation Fund of the Science and Technology Commission of Shanghai Municipality (15540723400), Open Foundation of State Key Laboratory for Modification of Chemical Fibers and Polymer Materials (LK1416) and Chinese Universities Scientific Fund (CUSF-DH-D-2015043).References
- O. C. Farokhzad and R. Langer, ACS Nano, 2009, 3, 16–20 CrossRef CAS PubMed.
- A. A. Bhirde, B. V. Chikkaveeraiah, A. Srivatsan, G. Niu, A. J. Jin, A. Kapoor, Z. Wang, S. Patel, V. Patel, A. M. Gorbach, R. D. Leapman, J. S. Gutkind, A. R. H. Walker and X. Y. Chen, ACS Nano, 2014, 8, 4177–4189 CrossRef CAS PubMed.
- S. P. Sherlock, S. M. Tabakman, L. M. Xie and H. J. Dai, ACS Nano, 2011, 5, 1505–1512 CrossRef CAS PubMed.
- H. Y. Liu, D. Chen, L. L. Li, T. L. Liu, L. F. Tan, X. L. Wu and F. Q. Tang, Angew. Chem., Int. Ed., 2011, 50, 891–895 CrossRef CAS PubMed.
- H. Gong, L. Cheng, J. Xiang, H. Xu, L. Z. Feng, X. Z. Shi and Z. Liu, Adv. Funct. Mater., 2013, 23, 6059–6067 CrossRef CAS.
- S. Wang, Z. Teng, P. Huang, D. Liu, Y. Liu, Y. Tian, J. Sun, Y. Li, H. Ju, X. Chen and G. Lu, Small, 2015, 11, 1801–1810 CrossRef CAS PubMed.
- D. Jaque, L. M. Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. M. Rodriguez and J. G. Sole, Nanoscale, 2014, 6, 9494–9530 RSC.
- Q. W. Tian, M. H. Tang, Y. G. Sun, R. J. Zou, Z. G. Chen, M. F. Zhu, S. P. Yang, J. L. Wang, J. H. Wang and J. Q. Hu, Adv. Mater., 2011, 23, 3542–3547 CrossRef CAS PubMed.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef CAS PubMed.
- Q. W. Tian, F. R. Jiang, R. J. Zou, Q. Liu, Z. G. Chen, M. F. Zhu, S. P. Yang, J. L. Wang, J. H. Wang and J. Q. Hu, ACS Nano, 2011, 5, 9761–9771 CrossRef CAS PubMed.
- E. Carrasco, B. del Rosal, F. Sanz-Rodriguez, A. J. de la Fuente, P. H. Gonzalez, U. Rocha, K. U. Kumar, C. Jacinto, J. G. Sole and D. Jaque, Adv. Funct. Mater., 2015, 25, 615–626 CrossRef CAS.
- Z. B. Zha, X. L. Yue, Q. S. Ren and Z. F. Dai, Adv. Mater., 2013, 25, 777–782 CrossRef CAS PubMed.
- J. Zhou, Z. G. Lu, X. J. Zhu, X. J. Wang, Y. Liao, Z. F. Ma and F. Y. Li, Biomaterials, 2013, 34, 9584–9592 CrossRef CAS PubMed.
- X. H. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS.
- M. S. Yavuz, Y. Y. Cheng, J. Y. Chen, C. M. Cobley, Q. Zhang, M. Rycenga, J. W. Xie, C. Kim, K. H. Song, A. G. Schwartz, L. H. V. Wang and Y. N. Xia, Nat. Mater., 2009, 8, 935–939 CrossRef CAS PubMed.
- H. Yuan, A. M. Fales and T. Vo-Dinh, J. Am. Chem. Soc., 2012, 134, 11358–11361 CrossRef CAS PubMed.
- H. Jo, H. Youn, S. Lee and C. Ban, J. Mater. Chem. B, 2014, 2, 4862–4867 RSC.
- H. K. Moon, S. H. Lee and H. C. Choi, ACS Nano, 2009, 3, 3707–3713 CrossRef CAS PubMed.
- D. Q. Chen, C. Wang, X. Nie, S. M. Li, R. M. Li, M. R. Guan, Z. Liu, C. Y. Chen, C. R. Wang, C. Y. Shu and L. J. Wan, Adv. Funct. Mater., 2014, 24, 6621–6628 CrossRef CAS.
- D. Q. Chen, C. Wang, F. Jiang, Z. Liu, C. Y. Shu and L. J. Wan, J. Mater. Chem. B, 2014, 2, 4726–4732 RSC.
- J. T. Robinson, S. M. Tabakman, Y. Y. Liang, H. L. Wang, H. S. Casalongue, D. Vinh and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- J. H. Liu, J. G. Han, Z. C. Kang, R. Golamaully, N. N. Xu, H. P. Li and X. L. Han, Nanoscale, 2014, 6, 5770–5776 RSC.
- B. Radisavljevic and A. Kis, Nat. Nanotechnol., 2013, 8, 147–148 CrossRef CAS PubMed.
- Q. Y. He, Z. Y. Zeng, Z. Y. Yin, H. Li, S. X. Wu, X. Huang and H. Zhang, Small, 2012, 8, 2994–2999 CrossRef CAS PubMed.
- Z. Y. Zeng, Z. Y. Yin, X. Huang, H. Li, Q. Y. He, G. Lu, F. Boey and H. Zhang, Angew. Chem., Int. Ed., 2011, 50, 11093–11097 CrossRef CAS PubMed.
- D. J. Late, B. Liu, H. S. S. R. Matte, V. P. Dravid and C. N. R. Rao, ACS Nano, 2012, 6, 5635–5641 CrossRef CAS PubMed.
- S. S. Chou, B. Kaehr, J. Kim, B. M. Foley, M. De, P. E. Hopkins, J. Huang, C. J. Brinker and V. P. Dravid, Angew. Chem., Int. Ed., 2013, 52, 4160–4164 CrossRef CAS PubMed.
- T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Z. Shi, L. Z. Feng, B. Q. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433–3440 CrossRef CAS PubMed.
- W. Y. Yin, L. Yan, J. Yu, G. Tian, L. J. Zhou, X. P. Zheng, X. Zhang, Y. Yong, J. Li, Z. J. Gu and Y. L. Zhao, ACS Nano, 2014, 8, 6922–6933 CrossRef CAS PubMed.
- T. Liu, S. X. Shi, C. Liang, S. D. Shen, L. Cheng, C. Wang, X. J. Song, S. Goel, T. E. Barnhart, W. B. Cai and Z. Liu, ACS Nano, 2015, 9, 950–960 CrossRef CAS PubMed.
- S. G. Wang, K. Li, Y. Chen, H. R. Chen, M. Ma, J. W. Feng, Q. H. Zhao and J. L. Shi, Biomaterials, 2015, 39, 206–217 CrossRef CAS PubMed.
- J. Yu, W. Y. Yin, X. P. Zheng, G. Tian, X. Zhang, T. Bao, X. H. Dong, Z. L. Wang, Z. J. Gu, X. Y. Ma and Y. L. Zhao, Theranostics, 2015, 5, 931–945 CrossRef CAS.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int. Ed., 2010, 49, 6288–6308 CrossRef CAS PubMed.
- K. P. Garcia, K. Zarschler, L. Barbaro, J. A. Barreto, W. O'Malley, L. Spiccia, H. Stephan and B. Graham, Small, 2014, 10, 2516–2529 CrossRef CAS PubMed.
- W. Feng, L. Chen, M. Qin, X. J. Zhou, Q. Q. Zhang, Y. K. Miao, K. X. Qiu, Y. Z. Zhang and C. L. He, Sci. Rep., 2015, 5, 17422 CrossRef CAS PubMed.
- L. S. Qi, Y. Y. Guo, J. J. Luan, D. R. Zhang, Z. X. Zhao and Y. X. Luan, J. Mater. Chem. B, 2014, 2, 8361–8371 RSC.
- G. J. Guan, S. Y. Zhang, S. H. Liu, Y. Q. Cai, M. Low, C. P. Teng, I. Y. Phang, Y. Cheng, K. L. Duei, B. M. Srinivasan, Y. G. Zheng, Y. W. Zhang and M. Y. Han, J. Am. Chem. Soc., 2015, 137, 6152–6155 CrossRef CAS PubMed.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- W. Feng, X. J. Zhou, C. L. He, K. X. Qiu, W. Nie, L. Chen, H. S. Wang, X. M. Mo and Y. Z. Zhang, J. Mater. Chem. B, 2013, 1, 5886–5898 RSC.
- W. Feng, W. Nie, C. L. He, X. J. Zhou, L. Chen, K. X. Qiu, W. Z. Wang and Z. Q. Yin, ACS Appl. Mater. Interfaces, 2014, 6, 8447–8460 CAS.
- W. Feng, W. Nie, Y. H. Cheng, X. J. Zhou, L. Chen, K. X. Qiu, Z. G. Chen, M. F. Zhu and C. L. He, Nanomed-Nanotechnol, Biol, Med, 2015, 11, 901–912 CrossRef CAS PubMed.
- Y. Yong, L. J. Zhou, Z. J. Gu, L. Yan, G. Tian, X. P. Zheng, X. D. Liu, X. Zhang, J. X. Shi, W. S. Cong, W. Y. Yin and Y. L. Zhao, Nanoscale, 2014, 6, 10394–10403 RSC.
- G. L. Frey, R. Tenne, M. J. Matthews, M. S. Dresselhaus and G. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 2883–2892 CrossRef CAS.
- M. Virsek, M. Krause, A. Kolitsch, A. Mrzel, I. Iskra, S. D. Skapin and M. Remskar, J. Phys. Chem. C, 2010, 114, 6458–6463 CAS.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS.
- H. S. S. R. Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. R. Rao, Angew. Chem., Int. Ed., 2010, 49, 4059–4062 CrossRef CAS PubMed.
- W. Zhu, K. Liu, X. Sun, X. Wang, Y. Li, L. Cheng and Z. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 11575–11582 CAS.
- M. H. Nia, M. Rezaei-Tavirani, A. R. Nikoofar, H. Masoumi, R. Nasr, H. Hasanzadeh, M. Jadidi and M. Shadnush, Journal of Paramedical Sciences, 2015, 6, 96–105 Search PubMed.
- H. Q. Hao, Q. M. Ma, F. He and P. Yao, J. Mater. Chem. B, 2014, 2, 7978–7987 RSC.
- B. Xia, W. Y. Zhang, J. S. Shi and S. J. Xiao, J. Mater. Chem. B, 2014, 2, 5280–5286 RSC.
- L. L. Xie, W. J. Tong, D. H. Yu, J. Q. Xu, J. Li and C. Y. Gao, J. Mater. Chem., 2012, 22, 6053–6060 RSC.
- C. Xu, D. R. Yang, L. Mei, Q. H. Li, H. Z. Zhu and T. H. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12911–12920 CAS.
- Y. Qiu, Y. Liu, L. M. Wang, L. G. Xu, R. Bai, Y. L. Ji, X. C. Wu, Y. L. Zhao, Y. F. Li and C. Y. Chen, Biomaterials, 2010, 31, 7606–7619 CrossRef CAS PubMed.
- Y. Wang, K. Y. Wang, R. Zhang, X. G. Liu, X. Y. Yan, J. X. Wang, E. Wagner and R. Q. Huang, ACS Nano, 2014, 8, 7870–7879 CrossRef CAS PubMed.
- H. Park, J. Yang, J. Lee, S. Haam, I. H. Choi and K. H. Yoo, ACS Nano, 2009, 3, 2919–2926 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27822h |
| This journal is © The Royal Society of Chemistry 2016 |