Synthesis, screening and sensing applications of a novel fluorescent probe based on C-glycosides†
Abstract
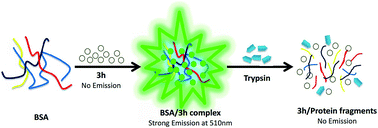
A novel water soluble fluorescent probe based on C-glycoside with an aromatic aldehyde unit has been synthesized and its UV/Vis and fluorescence spectra, aggregation and disaggregation with bovine serum albumin were studied. Meanwhile, the comparison between the carbohydrate-derived probe and other neutral probes indicated that neutral probes with a carbohydrate moiety showed excellent hydrophily and biocompatibility. The absorption and emission max wavelength of the arylvinyl ketone C-furyl glycoside in different solvents were in the range 402–437 and 479–549 nm respectively. Fluorescence spectroscopic studies showed that the as-prepared dye could be used in the labeling of BSA and monitoring trypsin hydrolysis as it displayed a “turn-on” mechanism when the dye was incubated with protein in water. Compared to traditional probes, this neutral fluorescent probe has the advantages of being water soluble, easily synthesized and suitable for a wide range of applications involving biomolecules.

 Please wait while we load your content...
Please wait while we load your content...