Tailoring SWIR emission in tri-lanthanide-doped CaF2 nanoparticles†
Xinyu Zhao and
Mei Chee Tan*
Engineering Product Development, Singapore University of Technology and Design, Singapore, 8 Somapah Road, Singapore 487372. E-mail: meichee.tan@sutd.edu.sg
First published on 8th February 2016
Abstract
Erbium (Er) doped CaF2 nanophosphors with enhanced short-wavelength infrared light (SWIR) emission synthesized in this work have promising potential for biomedical applications in diagnostics and deep tissue imaging due to their low vibrational energy, large optical transparency and superior biocompatibility. For deep tissue imaging application, it is critical to achieve highly emissive SWIR emitting CaF2 nanophosphors to enhance detection resolution and depth using the “tissue-transparent window”. In this work, we demonstrate substantial enhancement in the IR emission of CaF2 nanocrystals using a tri-doping scheme of ytterbium (Yb), erbium (Er) and cerium (Ce). The effects of Er, Ce and Yb dopant concentration on the IR emission intensity was systematically studied and the optimum Er, Ce and Yb dopant concentration was estimated to be ∼2, ∼10 and ∼20 mol%, respectively. At Yb 20 mol%, the IR emission intensity was increased ∼12 fold by optimizing the Ce dopant concentration.
1. Introduction
Infrared (IR)-emitting rare-earth (RE) doped materials are commonly used to fabricate the active optical components in photonic applications like solid-state lasers, optoelectronics, remote sensing and optical communications.1–5 A recent emerging application of IR-emitting RE doped nanophosphors for diagnostics and deep tissue imaging has been accelerated with the advent of commercially available short-wavelength infrared light (SWIR) emitting cameras.6–12 Several factors including spatial resolution, penetration depth as well as the biocompatibility of nanoprobes are critical for first-rate biomedical imaging. SWIR light spanning 1000–2300 nm have been demonstrated to be superior for biomedical imaging due to its lower absorption by tissue components, resulting in deeper penetration depth reaching up to 10 mm compared to the 1 mm limit of visible light.9 SWIR-emitting nanoprobes are able to fully exploit the infrared tissue-transparent window leading to advancements in the signal-to-noise ratio and resolution of biomedical imaging.7 In particular, erbium (Er) doped nanophosphors with an emission at ∼1530 nm which is within the “tissue-transparent window” shows the greatest promise.Calcium fluoride (CaF2) is stable, non-hygroscopic and has been widely used as the host material for RE doping materials.13–16 In particular, CaF2 has low vibrational energy (280 cm−1), which inhibits non-radiative losses leading to high internal quantum efficiency and bright emissions.17 It also has large optical transparency range (∼0.15 μm to 9 μm), which minimizes the re-absorption of infrared emitted light. These advantages of CaF2 make it suitable as a RE doped host material for preparation of highly emissive SWIR nanophosphors for biomedical applications. In addition, calcium and fluoride ions are common endogenous compounds and lattice substituents of calcified tissues (i.e., bone and teeth).18 Therefore, CaF2 has been demonstrated to have good biocompatibility which facilitates its application in optical bioimaging.19,20
Typically, Er doped nanophosphors not only emit IR emission at ∼1530 nm but also emit visible emissions at ∼540 nm and ∼650 nm upon excitation at 975 nm. These up-converted visible emissions that typically accompany the preferred IR emissions reduce the intensity of IR emission and subsequently limit the biomedical imaging sensitivity. One of the methods to facilitate the ∼1530 nm emission is to tailor the branching ratio by co-doping with cerium (Ce) ions.21 The energy difference in the lowest resonant transition of Ce ions (2F5/2 → 2F7/2) is coupled with the 4I11/2 → 4I13/2 transition of Er ions which facilitates the phonon-assisted energy transfer between Er and Ce ions to increase the IR emission efficiency and simultaneously decreases the upconversion losses.21–23 Upon Ce co-doping, the branching ratio was increased from ∼0.1–0.2 to ∼0.8–0.9.21–23 In addition, ytterbium (Yb) ions are usually employed as a co-doped sensitizer to increase the emission intensity of Er ions, whose high absorption cross-section and resonant energy level with Er ions facilitate the energy transfer between Yb and Er ions. Hence, the tri-doping of Yb, Ce and Er ions in CaF2 host would likely lead to bright, IR-emitting nanophosphors.
In this work, we report on the significant enhancement in IR emission of CaF2 nanocrystals using a tri-dopant scheme of Yb, Er and Ce. The optical behavior of CaF2 nanocrystals doped by a wide Ce dopant concentration range from 0 to 50 mol% was studied. The effects of Yb dopant concentration on the optical properties were also investigated. The optimum doping concentration of Er, Ce and Yb for IR emission was estimated to be ∼2, ∼10 and ∼20 mol%, respectively. At Yb 20 mol%, the IR emission intensity was increased ∼12 fold by optimizing the Ce dopant concentration. The brightly SWIR-emitting CaF2 nanocrystals synthesized in this work would potentially serve as efficient nanoprobes for biomedical imaging in the infrared tissue-transparent window.
2. Experimental section
2.1. Synthesis
All chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO) and used as received without any further purification. The CaF2:Yb,Er,Ce nanoparticles were prepared by using the hydrothermal method. Typically, stoichiometric amounts of calcium chloride (CaCl2, 96%), ytterbium(III) nitrate (Yb(NO3)3, 99.9%), erbium(III) nitrate (Er(NO3)3, 99.9%) and cerium(III) nitrate (Ce(NO3)3, 99.99%) were mixed together with 0.8 g NaF and 40 mL deionized water. The total amount of metal ions in the solution was 2 mmol. For example, to synthesize the sample of CaF2:Yb20Er2Ce10, 1.36 mmol CaCl2, 0.4 mmol Yb(NO3)3, 0.04 mmol Er(NO3)3 and 0.2 mmol Ce(NO3)3 were mixed together with 0.8 g NaF and 40 mL deionized water. The mixture was stirred for 1 h before it was transferred to a 125 mL Teflon liner and heated to 180 °C for 12 h. The obtained particles were washed by centrifugation and redispersion in deionized water for three times. After drying in oven at 60 °C, the obtained powders were used for further characterization.2.2. Material characterization
Particle size and morphology was observed using a JEOL 2010 transmission electron microscope operating at an acceleration voltage of 200 kV. X-ray powder diffraction (XRD) patterns were measured on a D8 Eco Advance powder diffractometer (Bruker AXS Inc., Madison, WI) using Cu Kα radiation with wavelength of 1.5418 Å, step size of 0.02° and duration of 0.5 s.Steady state luminescence spectra were measured upon excitation with a 975 nm continuous wave laser (CNI MDL-III-975, Changchun New Industries Optoelectronics Tech. Co. Ltd, China) using a FLS980 Fluorescence Spectrometer (Edinburgh Instruments Ltd., U.K.) with step size 2 nm and dwell time 0.2 s. Visible emissions were detected using R928P single photon counting photomultiplier in Peltier cooled housing (Edinburgh Instruments Ltd., U.K.). The IR emission was detected using Hamamatsu H10330A-75 photomultiplier in fan assisted TE cooled housing (Edinburgh Instruments Ltd., U.K.). The powder samples were packed in demountable Spectrosil far UV quartz Type 20 cells (Starna Cells, Inc., Atascadero, CA) with 0.5 mm path lengths for emission collection. The optical path for all photoluminescence measurements is kept constant. All luminescence measurements were measured three times and the average curves were shown in this study. To measure the time-resolved luminescence spectrum, the excitation source was modulated using an electronic pulse modulator to obtain excitation pulse at pulse duration of 5 μs with a repetition rate of 10 Hz. The pulse characteristics were measured using an oscilloscope (RTM 2052, Rohde&Schwarz Ltd.).
3. Results and discussion
3.1. Comparative dopant effects on CaF2 crystallization
Dopant chemistry (i.e., dopant concentration and types) has a significant impact on the optical properties of RE doped materials. In this study, wide dopant concentration ranges of Er, Ce and Yb were used to evaluate the effects of these dopants on the crystallization and optical behavior of CaF2 nanoparticles. Before adding any Ce dopant into CaF2 nanoparticles, the effect of Er dopant on the crystallization and optical property of CaF2:Yb,Er nanoparticles was studied. The CaF2 nanoparticles doped with 20 mol% Yb and different Er concentration of 0.5, 1, 2 and 5 mol% were synthesized and the corresponding XRD patterns are shown in Fig. S1.† All patterns were found to comprise of purely cubic CaF2 phase only (JCPDS 35-0816). Using the Scherrer's equation, the estimated grain sizes for the CaF2 samples are ∼20 nm for the entire Er dopant range (Fig. S2†).The CaF2 nanocrystals tri-doped with Yb, Er and Ce were prepared using the hydrothermal method. The morphology and size of as-synthesized CaF2 samples were characterized by TEM, as shown in Fig. 1a and b. Without the addition of any Ce dopant, uniform CaF2 nanocubes with an average particle size of ∼25.7 ± 5.1 nm were formed (Fig. 1a and S3a†). In contrast, smaller and equiaxed CaF2 nanospheres with an average particle size of ∼23.0 ± 5.5 nm are observed at Ce dopant concentration 10 mol% (Fig. 1b and S3b†). The observed change in CaF2 particle morphology from nanocube to nanosphere upon Ce doping was attributed to the effect of Ce ions on the crystallization and growth of CaF2 nanoparticles. The observed change in particle morphology with changing dopant concentration is also consistent with our earlier observations using NaYF4 as a host.21
A wide Ce dopant concentration range was used to evaluate the effects of Ce doping on the optical behavior of CaF2 nanoparticles doped with 20 mol% Yb and 2 mol% Er. Fig. 1c shows that the XRD patterns of as-synthesized CaF2 samples and the reference powder diffraction file for cubic CaF2 (JCPDS 35-0816). The observed broad peaks of CaF2 samples indicate that they have very small crystal sizes. Using the Scherrer's equation, the estimated grain sizes for the CaF2 samples are ∼10 to 20 nm over the range of Ce dopant concentrations (Fig. 1e). The estimated grain size generally decreases with the increase of Ce dopant concentration. In addition, an evident peak shift of up to ∼0.3° towards lower diffraction angles was observed with increasing Ce concentrations. This observed peak shift of ∼0.3° would correspond to a unit cell expansion of ∼0.06 Å based on calculations of the lattice parameter of CaF2 using Bragg's law and its (111) plane. The Yb3+, Er3+ and Ce3+ dopant ions are expected to occupy the Ca2+ sites which has a coordination number of 8 in the CaF2 lattice. This substitution of Ca by RE ions would result in some distortion (i.e., expansion or contraction) of the CaF2 crystal cell.24 Considering that the ionic radii of Yb3+ (0.985 Å) and Er3+ (1.004 Å)25 are smaller than that of Ca2+ ions (1.12 Å), we would expect some contraction of the CaF2 crystal upon doping with Yb and Er. In contrast, cell expansion would likely occur upon doping with the larger Ce ions (1.143 Å).25 Consequently, we have attributed the observed peak shift to the substitution of Ca2+ ions (1.12 Å) by larger Ce3+ ions (1.143 Å, size difference of ∼0.023 Å) within the host lattice. Due to the difference in charge for Ca2+ and Ce3+ ions, Ca2+ ion vacancies or interstitial F− ions are likely formed in order to maintain electroneutrality of CaF2 nanocrystal after substitution of Ca2+ sites by Ce3+ ions. EDX measurements were made to provide a preliminary estimate for these defects (Table S1†). Since the reduction in amount of Ca ions after Ce doping is larger than the Ce dopant amount, this suggests that the Ca ion vacancies are likely to exist in the Ce doped CaF2 nanocrystals. Similarly, the increase in the amount of F ions upon Ce doping indicates that the interstitial F ions were formed as well.
Fig. 1c also shows that a single cubic CaF2 phase was synthesized under relatively low Ce dopant concentrations of ≤10 mol%. In contrast, two additional phases of CeF3 (JCPDS 08-0045) and NaYbF4 (JCPDS 77-2043) are observed at higher Ce dopant concentrations of 20 and 50 mol%. The observation of an additional CeF3 phase demonstrates that the maximum Ce doping concentration in CaF2 should be less than 20 mol%. In our experiment, NaF was used as the fluorine source to synthesize CaF2 nanoparticles. Therefore, the formation of NaYbF4 phase was ascribed to the reaction between Yb ions and NaF precursor. Due to the low amounts of three phases of CaF2, CeF3 and NaYbF4 and significant peak overlaps between CaF2 and CeF3 phases, we were not able to further analyze XRD data using Rietveld analysis to obtain a quantitative amount of each phase. However, by analyzing 2θ peak at 45.2° for CeF3 and 30.1° for NaYbF4, a qualitative assessment of the extent of phase separation was conducted. The intensity of CeF3 for CaF2–Ce50 was increased by ∼39.3% compared to that of CaF2–Ce20, indicating that more CeF3 were segregated at the higher Ce concentration of 50 mol% (Fig. 1c, marked as ♣). In contrast, the intensity of NaYbF4 for CaF2–Ce20 is about two times of that of CaF2–Ce50 (Fig. 1c, marked as ♦), which demonstrates that phase separation of NaYbF4 occurred at a smaller extent at higher Ce concentrations. The phase separation phenomenon at high Ce dopant concentrations shows that Ce doping has an effect on the crystallization of CaF2 nanoparticles and subsequently also NaYbF4 phase segregation.21 At low Ce dopant concentrations, both Ce and Yb dopants are able to be successfully doped within the crystal lattice of CaF2 host. In contrast, at high Ce dopant concentrations, the competition doping of the two dopants into CaF2 host resulted in separate Ce and Yb crystallization to form different amounts of CeF3 and NaYbF4, respectively.
The phase separation phenomenon during CaF2 crystallization at high Ce concentration in aqueous environment is consistent with the results for NaYF4 reported in our previous work.21 The phase separation was also observed for NaYF4 crystallization when doping high concentrations of Ce using the hydrothermal method. The difference in solubility of the different RE ions (i.e., Yb, Er and Ce) in aqueous solution and their preferential solubility in host crystals affected the homogenous nucleation of NaYF4 or CaF2 in aqueous solution, which subsequently led to phase separation and limited the maximum dopant concentration in both hosts. However, no phase separation was observed for NaYF4 prepared using thermal decomposition method in oil phase solution and Ce dopant limit was increased up to 50 mol% in pure phase hexagonal NaYF4 via a homonucleation mechanism.21 Therefore, it is noted that reaction solvent plays an important role on the uniform nucleation of host materials and subsequently affects the dopant limit in host crystals.
Yb ions are usually added as a co-doped sensitizer to increase the adsorption at 975 nm and consequently the emission intensity of Er ions. The high absorption cross-section of Yb ions significantly enhances energy transfer to Er ions upon excitation at ∼975 nm through the resonance in energy between 2F5/2 level of Yb ions and 4I11/2 level of Er ions. Therefore, the concentration of Yb ions would likely affect the IR emission of the CaF2 nanocrystals.
To determine the optimum Yb dopant concentration, the CaF2 nanocrystals with 2 mol% Er and 10 mol% Ce and different Yb concentrations were synthesized and characterized using XRD (Fig. 1d). The CaF2 nanocrystals with Yb concentration at 10 and 20 mol% consist of a single cubic CaF2 phase (JCPDS 35-0816) while a second phase of NaYbF4 (JCPDS 77-2043) is observed for Yb concentration at 30 mol%. The formation of NaYbF4 phase at high Yb concentration suggests that the maximum Yb doping concentration in CaF2 should be less than 30 mol%. No CeF3 phase separation was observed for all three Yb concentrations, indicating that all the Ce ions are doped within the lattice of CaF2 or NaYbF4. Using the Scherrer's equation, the estimated grain sizes for CaF2 nanocrystals with different Yb doping concentrations were 14.6 ± 1.8, 15.8 ± 2.8 and 15.4 ± 3.0 nm, respectively (Fig. 1f).
3.2. Comparative dopant effects on IR emission
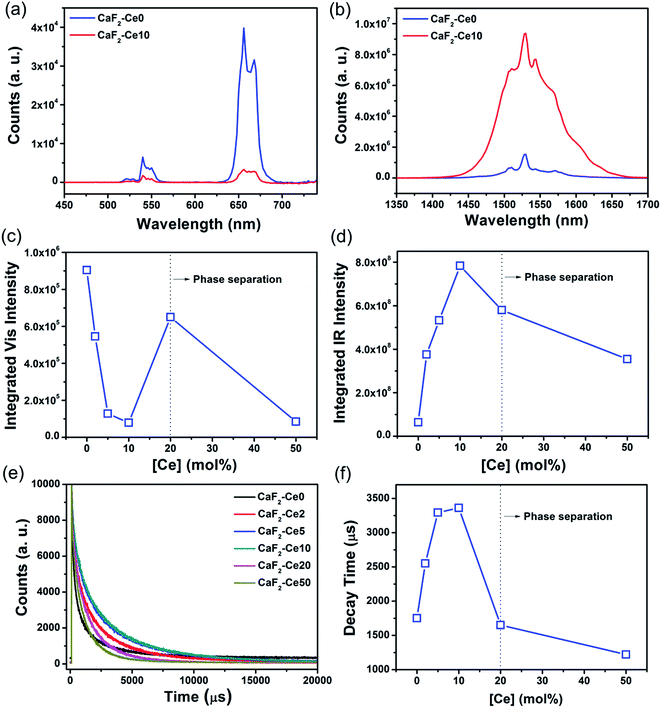
The optical characteristics of CaF2 nanocrystals only doped with Yb and Er was studied. Fig. 2 shows the typical emission spectra spanning visible and IR regions for CaF2:Yb,Er nanocrystals. The characteristic emission peaks were attributed to the 2H11/2 → 4I15/2 (∼525 nm), 4S3/2 → 4I15/2 (∼544 nm), 4F9/2 → 4I15/2 (∼658 nm) and 4I13/2 → 4I15/2 (∼1535 nm) transitions of Er3+ ions. To better compare the photoluminescent intensity, the integrated visible (green-plus-red, Fig. 2c) and IR (Fig. 2d) emission intensities were estimated using Origin© and subsequently plotted with respect to Er dopant concentration. The integrated visible emission intensity increased with Er dopant concentration from 0.5 to 2 mol% before decreasing as Er dopant concentration was increased to 5 mol%. The integrated IR emission intensity shows an identical trend to that of the integrated visible emission intensity. Therefore, at Yb of 20 mol%, the optimum Er dopant concentration was determined to be 2 mol%. The CaF2:Yb20Er2 sample with optimum optical emissions was selected as a host for the study of Ce dopant effects on the optical property.The photoluminescence emission spectra of CaF2:Yb20Er2 nanocrystals doped with different Ce concentrations obtained upon excitation at 975 nm were systematically investigated and the results are summarized in Fig. 3. The typical emission spectrum spanning visible and IR regions of tri-doped CaF2 nanocrystals are shown in Fig. 3a and b. The photoluminescence spectra for all other CaF2 nanoparticles with Ce doping concentration ranging from 0 to 50 mol% are shown in Fig. S4 in the ESI.† One can see that the visible emissions of CaF2 nanocrystals were significantly reduced, while the IR emission significantly increased upon doping with 10 mol% of Ce. The enhanced IR emission indicated the efficient phonon-assisted energy transfer between Ce and Er ions. Phonons are a quantum of energy of a lattice vibration, which can be considered analogous to a photon of electromagnetic waves. If a large number of phonons are required to bridge the energy gap, non-radiative relaxation loss is less likely. Consequently, a low phonon energy host which leads to more phonon bridging the gap, can efficiently inhibit non-radiative losses and give high internal quantum efficiency and bright emissions.5 In our tri-doped CaF2 nanoparticles, the non-radiative energy transfer between the 4I11/2 → 4I13/2 transition of Er ions and the 2F5/2 → 2F7/2 transition of Ce ions, which occurs in the form of phonon-assisted energy transfer, facilitates the IR emission enhancement.23
To further analyze the effects of Ce doping on the emission intensities, the integrated intensity of both visible emissions and IR emissions were calculated using Origin© as shown in Fig. 3c and d. The integrated intensity of visible emissions reduces rapidly upon the introduction of Ce. From 0 to 10 mol%, the integrated visible emission intensity decreases monotonically, when no phase separation of CeF3 or NaYbF4 was observed (Fig. 3c). At Ce dopant concentration of 10 mol%, the integrated visible intensity reaches a minimum point, where it decreased by ∼91% when compared to CaF2:Yb,Er (no Ce dopant). This evident reduction in integrated visible intensity can be attributed to the efficient inhibition of the upconversion pathways by phonon-assisted energy transfer between Ce and Er ions. The phonon-assisted energy transfer changes the branching ratios by altering the upconversion pathways and suppressing the visible emissions.
However, the integrated visible intensity increases significantly at higher Ce dopant concentration of 20 mol%, when phase separation of CeF3 and NaYbF4 occurred. Since some of the added Ce separated to form CeF3 phase, the actual amount of Ce incorporated within CaF2 lattice is likely to be at some value less than 20 mol%. Consequently, the lower concentration of Ce within CaF2 reduces the phonon-assisted energy transfer. In addition, NaYbF4, another host in which Er ions can be easily doped resulting in strong upconversion, was also formed when 20 mol% of Ce added within the reaction mixture.26,27 Consequently, some Er ions would most likely be doped within the NaYbF4 phase where visible emissions are possible resulting in the observed increased the integrated visible intensity. Further increase of Ce to 50 mol% resulted in the reduction of the integrated visible intensity to a value similar to that obtained for 10 mol% of Ce. Based on the XRD results, there was less NaYbF4 but more CeF3 phase at Ce of 50 mol%. The decreased NaYbF4 amounts limited the increase in visible emission intensities. It should be noted that, although Er could also be doped within CeF3, it was not likely that the observed increase in visible emission intensities (for Ce ≥20 mol%) was caused by the presence of CeF3 formation. CeF3 phase would not contribute to visible emissions due to the enhanced phonon-assisted energy transfer between Ce and Er ions which suppresses the upconversion pathways.23
The relationship between integrated IR emission intensities and Ce dopant concentration are shown in Fig. 3d. The integrated IR intensity shows a parabolic trend with a maximum at 10 mol%. The maximum point for IR emission corresponds with the minimum point of visible emissions as discussed earlier. As the Ce dopant amount increases from 0 to 10 mol%, the integrated IR emission intensity increased by ∼12 fold at 10 mol% of Ce. The significant enhancement in IR emission intensity is due to the phonon-assisted energy transfer processes associated with the 2F5/2 → 2F7/2 transition of Ce.22,28,29 The phonon-assisted energy transfer process efficiently increases the population of 4I13/2 energy state, which subsequently leads to the increase in the 4I13/2 → 4I15/2 (∼1535 nm) transition of Er ions. With the further increase of Ce dopant concentration from 10 to 50 mol%, the IR emission intensity decreases due to the concentration quenching. Concentration quenching is determined mainly by the dipole–dipole interaction between rare earth ions. The quenching effects vary according to R−6, where R is the interionic distance between the rare earth ions (e.g., the emitting ions). The luminescence is completely quenched for ions separated at a distance shorter than R, whereas ions separated by a distance longer R is not subjected to complete quenching. The typical critical interionic distance where concentration quenching occurs is approximately between 0.5 to 2 nm for a range of different host systems.7 Increasing the Ce dopant amounts within the CaF2 lattice changes the interionic distance between each of the rare earth pairs (e.g., Ce–Er pair, Ce–Ce or Er–Er pairs). As the distances decrease with the addition of Ce, the concentration quenching phenomenon dominates. The optimum Ce dopant concentration for IR emission is estimated to be ∼10 mol%, where a 12 times increment of IR emission intensity is achieved.
To further characterize the emission behavior of CaF2 nanocrystals doped with different amount of Ce, the fluorescence decay curves for the 1530 nm emission were measured as shown in Fig. 3e. To obtain decay constants, these fluorescence decay curves were analyzed by fitting to a double exponential equation,
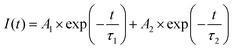 | (1) |
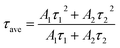 | (2) |
The average decay times and decay constants are shown in Fig. 3f, Tables 1 and S2.† The decay time is usually used to analyze contributions of both radiative and non-radiative relaxation pathways from a particular excited state. In general, a long decay time indicates bright emissions, low non-radiative losses, and high internal quantum efficiency. For the CaF2 samples, the plot of fitted decay time for 1530 nm emission with different Ce dopant concentration shows a parabolic relationship where the peak value is obtained at Ce concentration of 10 mol% (Fig. 3f). The Ce concentration for the observed peak value for the decay time is consistent to that observed for the integrated IR emission intensity (Fig. 3d).
| Sample | Decay time (μs) | Sample | Decay time (μs) |
|---|---|---|---|
| CaF2:Yb20Er2Ce0 | 1751.5 | CaF2:Er2Ce10Yb10 | 3341.0 |
| CaF2:Yb20Er2Ce2 | 2550.9 | CaF2:Er2Ce10Yb20 | 3372.4 |
| CaF2:Yb20Er2Ce5 | 3293.6 | CaF2:Er2Ce10Yb30 | 2309.3 |
| CaF2:Yb20Er2Ce10 | 3372.4 | ||
| CaF2:Yb20Er2Ce20 | 1650.3 | ||
| CaF2:Yb20Er2Ce50 | 1220.5 |
The estimated decay time increases first from ∼1751.5 μs for CaF2 without any Ce dopant to ∼3372.4 μs for CaF2 with 10 mol% Ce. The significant increase of ∼92.5% in decay time was attributed to the more efficient radiative transitions achieved by the enhanced energy transfer associated with the 2F5/2 → 2F7/2 transition of Ce ions. Subsequently, the decay time decreases from ∼3372.4 to 1220.5 μs as Ce dopant concentration increases from 10 to 50 mol%. The decreased in decay time was most likely due to the suppressed radiative transitions from the desired excited state as a result of concentration quenching. These measurements show that Ce doping can effectively improve the IR emission efficiency for CaF2 nanocrystals and the optimum Ce dopant concentration is 10 mol%.
The effects of Yb dopant concentration on both visible and IR emission intensities of CaF2 nanocrystals were also studied (see Fig. 4). When the Yb concentration was increased from 10 to 20 mol%, the decrease in integrated visible intensity was most likely due to the efficient inhibition of upconversion pathways through the phonon-assisted energy transfer between Ce and Er ions (see Fig. 4a and c). However, the integrated visible intensity increased upon further addition of Yb to 30 mol%, where an additional phase of NaYbF4 was also observed. The increase in visible emissions was likely from the increased population of 4I11/2 level of Er ions due to the enhanced energy transfer between the 2F5/2 level of Yb ions and 4I11/2 level of Er ions, which facilitates the upconversion pathways. The additional NaYbF4 phase also served as host for Er, which facilitated the upconversion pathways.
The IR emission intensity of CaF2 samples shows the opposite trend with increasing Yb dopant concentration compared to that for the visible emission (see Fig. 4b and d). The optimum Yb concentration was ∼20 mol%. When the Yb dopant concentration was less than 20 mol% the absorption of excitation light was lowered, while the concentration quenching reduced the IR emission intensity at high Yb concentration.
Fluorescence decay curves for the 1530 nm emission from CaF2 samples with different Yb dopant concentrations were measured (see Fig. 4e). The fluorescence decay curves were also fitted to a double exponential equation so as to obtain the average decay times and decay constants, as shown in Fig. 4f, Tables 1 and S2.† The decay time shows a slight increase from ∼3341.0 to 3372.4 μs with the increase in Yb concentration from 10 to 20 mol%, which is next followed by a substantial decrease to ∼2309.3 μs at 30 mol% of Yb (see Fig. 4f). Therefore, the optimum Yb dopant concentration based on the decay time measurements was 20 mol%, which is consistent with our earlier results (see Fig. 4d). At 20 mol% of Yb, efficient energy absorption and energy transfer to Er emitters led to the enhanced IR emission efficiency.
4. Summary
In summary, we have prepared CaF2 nanocrystals with enhanced IR emission using a tri-dopant scheme of Yb, Er and Ce ions. Tri-doped CaF2 nanocrystals with sizes of ∼23.0 ± 5.5 nm were synthesized using the hydrothermal method. The effects of Er, Ce and Yb dopant concentration on the IR emission intensity were systematically studied and the optimum Er, Ce and Yb dopant concentration was 2, 10 and 20 mol%, respectively. At 20 mol% of Yb, the IR emission intensity was increased by ∼12 fold after optimizing the Ce dopant concentration. The co-doping of Ce facilitated the energy transfer between Ce and Er ions to enhance the down-shifting IR emission and inhibit the up-conversion visible emissions. In particular, the additional CeF3 and NaYbF4 phases formed during CaF2 synthesis at high Ce concentrations (i.e., 20 and 50 mol%), led to the reduction in IR emission and increased visible emissions. The CaF2 nanocrystals with enhanced IR emission synthesized in this work are promising candidates in applications such as polymeric optical waveguides and SWIR imaging probes.Acknowledgements
MC Tan and XY Zhao would like to gratefully acknowledge the funding support from the Agency for Science, Technology and Research Public Sector Fund (Project number 13212056) and Singapore University of Technology and Design-Massachusetts Institute of Technology International Design Centre (SUTD-MIT IDC) (Project number IDG31400106). MC Tan and XY Zhao would also like to thank Y Lu for her assistance in making TEM measurements.References
- B. Simondi-Teisseire, B. Viana, D. Vivien and A. Lejus, Opt. Mater., 1996, 6, 267–274 CrossRef CAS.
- A. Jha, Asia-Pacific Remote Sensing Symposium, International Society for Optics and Photonics, 2006, 6406, 640918-1-12 Search PubMed.
- A. Ikesue, Y. L. Aung, T. Taira, T. Kamimura, K. Yoshida and G. L. Messing, Annu. Rev. Mater. Res., 2006, 36, 397–429 CrossRef CAS.
- A. Kenyon, Prog. Quantum Electron., 2002, 26, 225–284 CrossRef CAS.
- M. C. Tan, D. J. Naczynski, P. V. Moghe and R. E. Riman, Aust. J. Chem., 2013, 66, 1008–1020 CrossRef CAS.
- M. Zevon, V. Ganapathy, H. Kantamneni, M. Mingozzi, P. Kim, D. Adler, Y. Sheng, M. C. Tan, M. Pierce and R. E. Riman, Small, 2015, 11, 6347–6357 CrossRef CAS PubMed.
- B. van Saders, L. Al-Baroudi, M. C. Tan and R. E. Riman, Opt. Mater. Express, 2013, 3, 566–573 CrossRef CAS.
- G. A. Hebbink, J. W. Stouwdam, D. N. Reinhoudt and F. C. van Veggel, Adv. Mater., 2002, 14, 1147–1150 CrossRef CAS.
- D. J. Naczynski, M. C. Tan, M. Zevon, B. Wall, J. Kohl, A. Kulesa, S. Chen, C. M. Roth, R. E. Riman and P. V. Moghe, Nat. Commun., 2013, 4, 2199 CAS.
- Y. Sheng, L.-D. Liao, N. Thakor and M. C. Tan, Sci. Rep., 2014, 4, 6562 CrossRef CAS PubMed.
- J. Shen, G. Chen, A. M. Vu, W. Fan, O. S. Bilsel, C. C. Chang and G. Han, Adv. Opt. Mater., 2013, 1, 644–650 CrossRef.
- R. Wang, X. Li, L. Zhou and F. Zhang, Angew. Chem., 2014, 126, 12282–12286 CrossRef.
- S. Q. Huang, L. Gu, C. Miao, Z. Y. Lou, N. W. Zhu, H. P. Yuan and A. D. Shan, J. Mater. Chem. A, 2013, 1, 7874–7879 CAS.
- A. Jouini, A. Brenier, Y. Guyot, G. Boulon, H. Sato, A. Yoshikawa, K. Fukuda and T. Fukudat, Cryst. Growth Des., 2008, 8, 808–811 CAS.
- A. Lyberis, A. J. Stevenson, A. Suganuma, S. Ricaud, F. Druon, F. Herbst, D. Vivien, P. Gredin and M. Mortier, Opt. Mater., 2012, 34, 965–968 CrossRef CAS.
- X. M. Sun and Y. D. Li, Chem. Commun., 2003, 1768–1769 RSC.
- R. F. Barrow, D. A. Long and D. J. Millen, Molecular Spectroscopy, Royal Society of Chemistry, 1974 Search PubMed.
- G. Chen, J. Shen, T. Y. Ohulchanskyy, N. J. Patel, A. Kutikov, Z. Li, J. Song, R. K. Pandey, H. Ågren and P. N. Prasad, ACS Nano, 2012, 6, 8280–8287 CrossRef CAS PubMed.
- C. Feldmann, M. Roming and K. Trampert, Small, 2006, 2, 1248–1250 CrossRef CAS PubMed.
- H. J. Moon, K. N. Kim, K. M. Kim, S. H. Choi, C. K. Kim, K. D. Kim, R. Z. LeGeros and Y. K. Lee, J. Biomed. Mater. Res., Part A, 2005, 74, 497–502 CrossRef PubMed.
- X. Zhao and M. C. Tan, J. Mater. Chem. C, 2015, 3, 10207–10214 RSC.
- Z. Meng, T. Yoshimura, K. Fukue, M. Higashihata, Y. Nakata and T. Okada, J. Appl. Phys., 2000, 88, 2187–2190 CrossRef CAS.
- M. C. Tan, G. Kumar and R. E. Riman, Opt. Express, 2009, 17, 15904–15910 CrossRef CAS PubMed.
- L. P. Singh, S. K. Srivastava, R. Mishra and R. S. Ningthoujam, J. Phys. Chem. C, 2014, 118, 18087–18096 CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, A32, 751–767 CrossRef CAS.
- Z. Yi, S. Zeng, W. Lu, H. Wang, L. Rao, H. Liu and J. Hao, ACS Appl. Mater. Interfaces, 2014, 6, 3839–3846 CAS.
- O. Ehlert, R. Thomann, M. Darbandi and T. Nann, ACS Nano, 2008, 2, 120–124 CrossRef CAS PubMed.
- S. Shen, B. Richards and A. Jha, Opt. Express, 2006, 14, 5050–5054 CrossRef CAS PubMed.
- X. S. Zhai, J. Li, S. S. Liu, X. Y. Liu, D. Zhao, F. Wang, D. M. Zhang, G. S. Qin and W. P. Qin, Opt. Mater. Express, 2013, 3, 270–277 CrossRef CAS.
- M. C. Tan, G. Kumar, R. E. Riman, M. Brik, E. Brown and U. Hommerich, J. Appl. Phys., 2009, 106, 063118 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26233j |
| This journal is © The Royal Society of Chemistry 2016 |