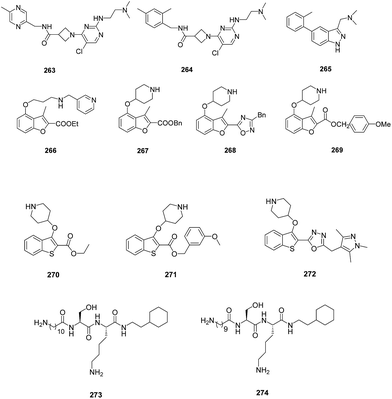
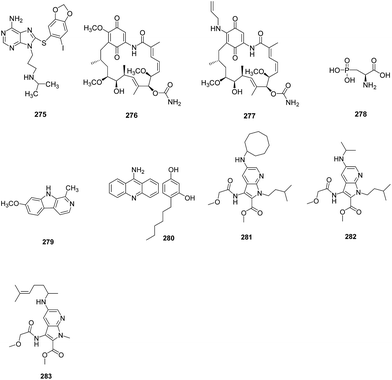
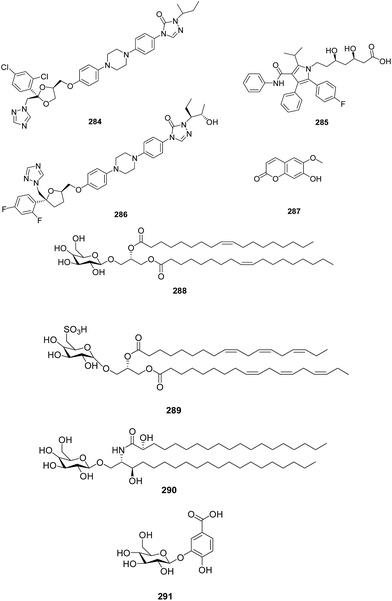
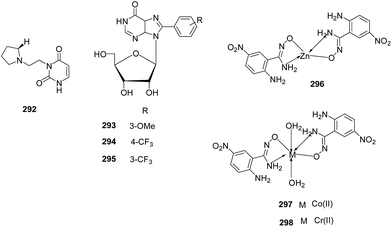
A structure guided drug-discovery approach towards identification of Plasmodium inhibitors
Babita Aneja
a,
Bhumika Kumar
a,
Mohamad Aman Jairajpuri
b and
Mohammad Abid
*a
aMedicinal Chemistry Lab, Department of Biosciences, Jamia Millia Islamia (A Central University), Jamia Nagar, New Delhi 110025, India. E-mail: mabid@jmi.ac.in; Fax: +91-11-26980229; Tel: +91-8750295095
bProtein Conformation and Enzymology Lab, Department of Biosciences, Jamia Millia Islamia (A Central University), Jamia Nagar, New Delhi 110025, India
First published on 4th February 2016
Abstract
Rapidly increasing resistance to the currently available antimalarial drugs has drawn attention globally for the search for more potent novel drugs with a high therapeutic index. The genome sequencing of the human malarial parasite Plasmodium falciparum has provided extensive information to understand potential target pathways and efforts are being made to develop lead inhibitors with a hope to eliminate the disease. This review is focused on a brief description of key biochemical targets identified from the genome sequence of P. falciparum. This review also summarizes the work undertaken by different scientific groups over the last five years to develop inhibitors from natural, semisynthetic or synthetic sources, which will be valuable to medicinal chemists to develop novel antimalarial agents.
Introduction
Despite global efforts to control and eradicate malaria, it is one of the most widespread and deadliest parasitic diseases affecting more than 40% of the world's population.1 The World Health Organization (WHO) factsheet for 2014 reveals more than 198 million cases with an estimated 584![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fatalities. The severity of this disease is prevalent in the WHO African region with an estimated 90% mortality rate, affecting mainly children under the age of 5, who account for 78% of all deaths.2 This disease is mainly attributable to five species of malaria parasite i.e. Plasmodium which are known to infect humans – P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. Out of these, P. falciparum and P. vivax are biggest threat to public health. While P. vivax is widely distributed geographically, P. falciparum is reportedly more fatal.3
000 fatalities. The severity of this disease is prevalent in the WHO African region with an estimated 90% mortality rate, affecting mainly children under the age of 5, who account for 78% of all deaths.2 This disease is mainly attributable to five species of malaria parasite i.e. Plasmodium which are known to infect humans – P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. Out of these, P. falciparum and P. vivax are biggest threat to public health. While P. vivax is widely distributed geographically, P. falciparum is reportedly more fatal.3
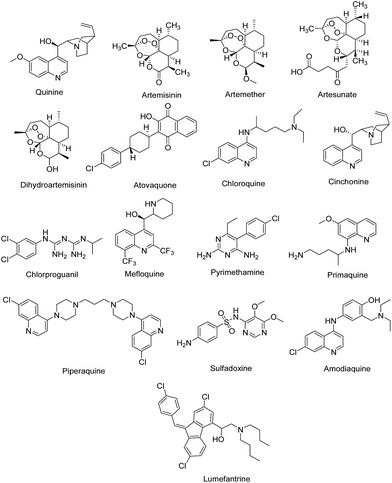
Though a number of antimalarial drugs such as quinine, artemisinin, artemether, artesunate, dihyroartemisinin, atovaquone, chloroquine, cinchonine, chlorproguanil, mefloquine, pyrimethamine, primaquine, piperaquine, sulfadoxine, amodiaquine, lumefantrine (Fig. 1) are available, these drugs are rapidly losing their therapeutic potential due to emerging drug resistance in Plasmodium.4,5 The parasitic mutations associated with resistance to antimalarial drugs primarily contributes to the re-emergence of the disease and its spread to new places and populations. Although artemisinin-based combination therapy has been recommended by WHO as the first-line of treatment for multi-drug resistant P. falciparum infection which is non-responsive to quinoline and antifolate based drugs,6 but the frequent use of artemisinin and its derivatives has also resulted in resistance to this class of drugs and the first signs of resistance have emerged in South-East Asian countries.2,7 Thus, there is an urgent need to develop new therapeutic agents with novel mode of action to save people from the grasp of this deadly disease and to reduce morbidity and mortality.
Different approaches adopted till now by the scientific communities for antimalarial drug discovery are mainly based upon (a) combination of existing drugs (artemether–lumefantrine, amodiaquine–artesunate, dihyroartemisinin–piperaquine) (b) chemical modification of known drugs and compound classes (mefloquine, primaquine, chloroquine, artesunate) (c) “piggy back approach” for the compounds active against other diseases (antifolates, tetracyclines, atovaquone) (d) use of natural products (quinine, artemisinin) (e) use of drug resistance reversers (verapamil, desipramine, trifluoperazine)8 (f) in vitro whole cell parasitic assay (KAE609 (formerly known as NITD609) and KAF156 under phase II clinical trials).9 The most recent approach is the identification and validation of novel molecular targets and the development of inhibitors active against these targets. The accessibility of whole genome sequence of P. falciparum has facilitated the identification of potential targets for the development of new drugs and also shed light on the mechanism of action of older drugs. The validation of identified target is crucial for this approach and is carried out by repeated antiparasitic activity of different lead compounds against that particular target. Identification of potentially active compounds and their optimization subsequently results in lead candidates that are further taken to pre-clinical and clinical investigations.10 Since the qualitative information of all the identified lead compounds and potential drug targets imparts impetus to the drug discovery programmes, this review aims to provide the same for antimalarial research boon. In this review, the antimalarial potential of various natural, semi-synthetic as well as synthetic compounds reported during the last 5 years (2010–2015) are compiled and discussed in target-oriented manner, which will provide a stand to medicinal chemists for the development of better antimalarial therapeutics.
Target insights of antimalarial inhibitors
Drug targets which have been identified and characterized against malaria can be classified on the basis of their locations within the malaria parasite. The enzymes that are crucial for parasite growth and survival are the strategic targets of these inhibitors. Inhibiting either of these may prove lethal to the parasite thus targeting these enzymes will be a powerful strategy in the malaria eradication campaign. The key targets and their inhibitors along with their locations in the parasite are summarised in Table 1.| Target location | Pathway/mechanism | Target enzyme | Inhibitors | References |
|---|---|---|---|---|
| Parasite invasion and release | Merozoite invasion | Apical membrane antigen 1 (AMA1) | N-Methylated 20-residue peptide, R1 | 198 |
| Merozoite egress | Subtilisin-like protease 1 (PfSUB1) | Chloroisocoumarin JCP104, difluorostatone based inhibitors | 20 and 199 | |
| Food vacuole | Hemoglobin degradation | Plasmepsin (PM) | Allophenylnorstatine-based inhibitor | 42 |
| Falcipain (FP) | Cyanopyrimidine based analogues | 45 | ||
| Aminopeptidase | Bestatin analogues, phosphonic acid arginine mimetics, hydroxamate derivatives | 88,89 and 91 | ||
| Dipeptidyl aminopeptidase (DPAP) | Dipeptide based analogues | 94 | ||
| Nucleus | Nucleic acid regulation | Histone deacetylase (HDAC) | Apicidin, WR301801, aryltriazolylhydroxamates based derivatives | 98, 200 and 201 |
| Cyclin dependent protein kinase (CDK) | Oxindole derivatives | 202 | ||
| Mitochondria | Electron transport system | Cytochrome bc1 complex | ELQ-300 | 113 |
| Dihydroorotate dehydrogenase (DHODH) | Phenyl-substituted triazolopyrimidines | 121 | ||
| NADH: ubiquinone oxidoreductase (PfNDH2) | SL-2-25 | 134 | ||
| Apicoplast | Type II fatty acid biosynthesis | PfFabZ | NAS91 | 203 |
| PfFabI | Coumarin-based triclosan analogues | 142 | ||
| PfFabG | (−)-Catechin gallate | 204 | ||
| Isoprenoid biosynthesis | DOXP reductoisomerase (DXR) | Fosmidomycin, FR-900098 | 150 | |
| Protein myristoylation | N-Myristoyltransferase (NMT) | Benzo[b]thiophene derivatives | 159 | |
| Cytosol | Protein synthesis | Heat shock protein 90 (Hsp 90) | Geldanamycin | 165 |
| Glycolysis | Lactate dehydrogenase (LDH) | Gossypol, azole derivatives | 205 and 206 | |
| Nucleic acid biosynthesis | Purine nucleoside phosphorylase (PNP) | Immucillins | 207 | |
| Folate metabolism | Dihydrofolate reductase (DHFR) | Pyrimethamine, cycloguanil | 208 | |
| Dihyropteroate synthase (DHPS) | Dapsone, sulphamethaoxazole | 209 | ||
| Thymidylate synthase (TS) | 5-Fluoroorotate | 210 | ||
| Carbon dioxide metabolism | Carbonic anhydrase (CA) | Acetazolamide, sulfanilamide | 211 | |
| Oxidative stress | Thioredoxin reductase | 5,8-Dihydroxy-1,4-naphthoquinone | 212 | |
| Pyrimidine biosynthesis | Thymidylate kinase (TMK) | Thymidine derivatives | 194 |
1. Parasite invasion and release
Plasmodium adopts multiple invasive ‘zoite’ forms to complete their multi-stage development in both the mosquito vector and the human host (Fig. 2).11 Female Anopheles mosquito injects Plasmodium sporozoites into the human host which move towards the liver where they invade hepatocytes to start liver stage infection. These intracellular parasites develop into exoerythrocytic merozoites which are released into the blood stream where they invade erythrocytes initiating disease's symptomatic erythrocyte stage.12 This process of merozoite invasion is facilitated by the apical membrane antigen 1 (AMA1) which is involved in the formation of moving junction in association with rhoptry neck protein (RON).13 Being obligate intracellular parasites, merozoites undergo maturation within the erythrocyte and develop cyclically through ring, trophozoite and schizont stage inside parasitophorous vacuole. At the final stage of development, parasite produces 16–32 daughter merozoites due to asexual division (schizogony) which are released upon egress to invade new erythrocytes mediated by cysteine protease dipeptidyl peptidase 3 (DPAP3) and subtilisin family serine protease PfSUB1.14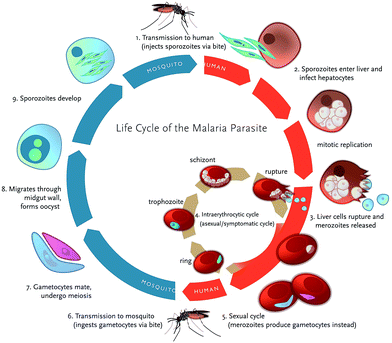 | ||
| Fig. 2 Life cycle of Plasmodium falciparum illustrating the various stages (reproduced with permission from Klein, 2013). | ||
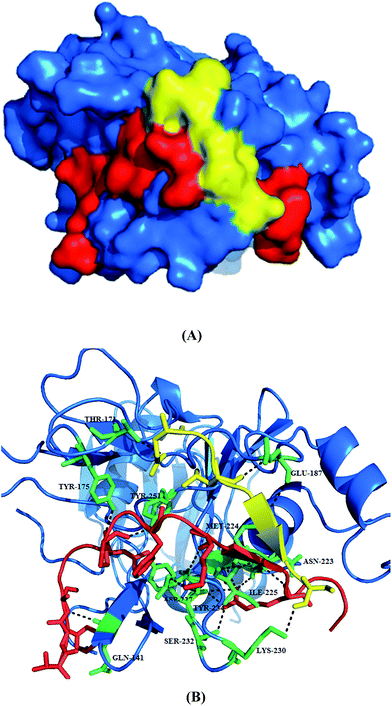 | ||
| Fig. 3 Structure of PfAMA1 complexed with R1 peptide. (A) The co-crystal structure of PfAMA1 (blue surface) with R1 shows two bound peptides, R1 major (red) and R1 minor (yellow). (B) Detailed analysis of interactions at the PfAMA1-R1 major, PfAMA1-R1 minor interfaces. The pictures are drawn using PyMol (PDB ID: 3SRJ). | ||
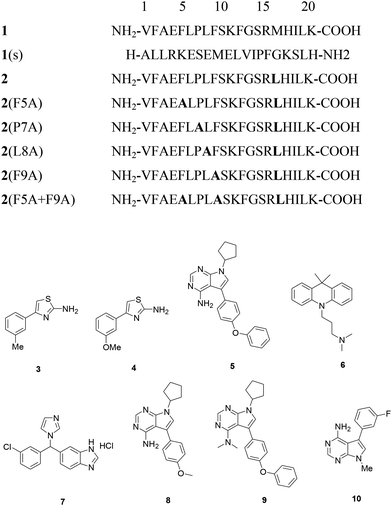
Key binding residues involved in the tight binding of peptide inhibitors to the AMA1 of P. falciparum were identified. The structural analogues were based on 1, a potent inhibitor of parasite invasion of erythrocytes in vitro. The importance of residues 5–10 present in the turn-like conformation of 1 (solution structure) for binding to AMA1 was analysed through site-directed mutagenesis and NMR spectroscopy. Both the 1 and modified peptide, R2 (2) exhibited similar binding affinities to PfAMA1, having IC50 values of 177 and 250 nM. The replacement of Met16 by Leu was not detrimental to the binding affinity of 2 suggesting that this residue is not critical for this interaction. Substitution of Phe5, Pro7, Leu8 and Phe9 with alanine led to significant decrease in the binding affinity (7.5- to >350-fold) for AMA1. Lack of significant changes in the chemical shifts for the backbone amide and CαH resonances of these mutant peptides in comparison to 2 suggests that these mutations caused little effect on the overall solution conformation of 2. Slightly large differences were observed for residues flanking position 7 in the 2(P7A). Based on R1 mimetics, the identification of nonpolar region possessing residues critical for AMA1 binding provides a platform for the designing of antimalarial agents.16
Fragment-based drug design approach was also employed for the development of small molecule inhibitors of PfAMA1. This approach which employed saturation transfer difference (STD) NMR and Carr–Purcell–Meiboom–Gill (CPMG) experiments identified fragments that bind to the hydrophobic cleft with the overall hit rate of 5%. The high hit rate obtained in this experiment strongly supports the presence of a druggable pocket within the hydrophobic cleft that is capable of binding to small molecules. The hits obtained via R1 competing experiments were reportedly grouped into 2-aminothiazoles and 2-arylfurans series. The R1-competing hits were evaluated against immobilized 3D7 PfAMA1 for estimation of binding affinities. Among 57 NMR hits evaluated, 46 compounds displayed binding in the surface plasmon resonance (SPR) experiments with binding affinities (KD values) weaker than 1 mM except two compounds and the active hits displayed KD value of 600 μM. The ligand efficiencies (LE) of these compounds were found between 0.2 and 0.3 kcal mol−1 heavy per atom while fragments with ligand efficiencies of ≥0.3 kcal mol−1 heavy per atom belonged to 2-aminothiazole series. The SAR studies around 4-aryl substituted 2-aminothiazoles revealed that meta substituted methyl, 3 (KD = 660 μM, LE = 0.33) and methoxy group substituted compound, 4 (KD = 1.2 mM, LE = 0.28) was responsible for high potency. Although these are weak binders of AMA1 but these may serve as building blocks for the development of potent AMA1 inhibitors with broad strain specificity.13
Several small-molecule inhibitors targeting AMA1–RON2 complex were identified by screening a ∼21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 member library via AlphaScreen assay by utilizing short RON2 peptide (RON2L). This screen further identified three compounds, NCGC00015280 (5), NCGC00014044 (6) and NCGC00181034 (7) (Fig. 4) that exhibited the tendency to block the interaction of AMA1 and RON2 as well as inhibited merozoite invasion of RBCs with IC50 values in the range of 21–29 μM. All the three compounds blocked merozoite invasion of four genetically distinct Pf clones with IC50 values of 10–14 μM with minimal to no effect on schizont rupture. Chemical modification of 5 led to identification of NCGC00262650 (8) and NCGC00262654 (9) which exhibited improved inhibition with IC50 values of 9.8 μM and 6.0 μM in comparison to resynthesized 5's activity of 30 μM. Combination of 5 and 8 with dihydroartemisinin (DHA) reportedly enhanced the efficiency of growth inhibition by simultaneous targeting of intracellular parasites and merozoite invasion. Such combinations provide an excellent strategy to prevent as well as treat artemisinin-resistant parasites. The mode of action of these inhibitors was identified by using SPR and depletion assays which is facilitated through binding to AMA1 and blocking its interaction with RON2 thus preventing the junction formation which leads to the inhibition of merozoite invasion. Molecular docking within the hydrophobic cleft also proposed the presence of two potential interaction sites (hotspots) for the small molecule inhibitors. One hotspot was located near the flexible loop region where a well-formed hydrophobic pocket is present adjacent to DII loop and is involved in extensive van der Walls and aromatic interactions with compounds 5, 8 and 9, resulting in prevention of AMA1–RON2 interaction. Other binding mode was present at a more open and rigid region along the hydrophobic groove which plays an important role in the RON2 peptide binding. Thus, the identification of these hotspots provides insights for the development of next generation AMA1–RON2 inhibitors.19
000 member library via AlphaScreen assay by utilizing short RON2 peptide (RON2L). This screen further identified three compounds, NCGC00015280 (5), NCGC00014044 (6) and NCGC00181034 (7) (Fig. 4) that exhibited the tendency to block the interaction of AMA1 and RON2 as well as inhibited merozoite invasion of RBCs with IC50 values in the range of 21–29 μM. All the three compounds blocked merozoite invasion of four genetically distinct Pf clones with IC50 values of 10–14 μM with minimal to no effect on schizont rupture. Chemical modification of 5 led to identification of NCGC00262650 (8) and NCGC00262654 (9) which exhibited improved inhibition with IC50 values of 9.8 μM and 6.0 μM in comparison to resynthesized 5's activity of 30 μM. Combination of 5 and 8 with dihydroartemisinin (DHA) reportedly enhanced the efficiency of growth inhibition by simultaneous targeting of intracellular parasites and merozoite invasion. Such combinations provide an excellent strategy to prevent as well as treat artemisinin-resistant parasites. The mode of action of these inhibitors was identified by using SPR and depletion assays which is facilitated through binding to AMA1 and blocking its interaction with RON2 thus preventing the junction formation which leads to the inhibition of merozoite invasion. Molecular docking within the hydrophobic cleft also proposed the presence of two potential interaction sites (hotspots) for the small molecule inhibitors. One hotspot was located near the flexible loop region where a well-formed hydrophobic pocket is present adjacent to DII loop and is involved in extensive van der Walls and aromatic interactions with compounds 5, 8 and 9, resulting in prevention of AMA1–RON2 interaction. Other binding mode was present at a more open and rigid region along the hydrophobic groove which plays an important role in the RON2 peptide binding. Thus, the identification of these hotspots provides insights for the development of next generation AMA1–RON2 inhibitors.19
To address the issues such as low solubility, high clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and low LLEAT values related to small molecule inhibitors of PfAMA1 reported previously by Srinivasan et al.,19 a critical evaluation was carried out for these compounds by Devine et al.15 by using their own method for monitoring AMA1 ligand binding. Compounds 5 and 9 exhibited extensive aggregation in comparison to 8 which was evidenced from the presence of very weak signals obtained in the 1H-NMR spectra. The evaluation of binding affinity of 8 to AMA1 by surface plasmon resonance (SPR) revealed unambiguous over-binding at higher concentrations while inconsistency was observed at lower concentrations with an affinity for AMA1 tighter than ∼1 mM. IC50 for compound 8 was found 9.8 μM as reported by Srinivasan et al.19 This discrepancy observed suggests that this series of compounds caused effects on host cell invasion by some other mechanism than direct inhibition of AMA1. To resolve the issues of solubility, another series of pyrrolo[2,3-d]pyrimidine-4-amines was synthesized and reportedly possess clog
P and low LLEAT values related to small molecule inhibitors of PfAMA1 reported previously by Srinivasan et al.,19 a critical evaluation was carried out for these compounds by Devine et al.15 by using their own method for monitoring AMA1 ligand binding. Compounds 5 and 9 exhibited extensive aggregation in comparison to 8 which was evidenced from the presence of very weak signals obtained in the 1H-NMR spectra. The evaluation of binding affinity of 8 to AMA1 by surface plasmon resonance (SPR) revealed unambiguous over-binding at higher concentrations while inconsistency was observed at lower concentrations with an affinity for AMA1 tighter than ∼1 mM. IC50 for compound 8 was found 9.8 μM as reported by Srinivasan et al.19 This discrepancy observed suggests that this series of compounds caused effects on host cell invasion by some other mechanism than direct inhibition of AMA1. To resolve the issues of solubility, another series of pyrrolo[2,3-d]pyrimidine-4-amines was synthesized and reportedly possess clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 0.93–2.35 with reduced aggregation behavior. These compounds were reported as weak inhibitors of PfAMA1 with KD values of ≥1 mM but displayed substantial inhibition of parasite growth suggesting alternate mechanism of action for these compounds. The best analogue 10 among the series displayed IC50 value of < 31 μM (Fig. 4).
P values of 0.93–2.35 with reduced aggregation behavior. These compounds were reported as weak inhibitors of PfAMA1 with KD values of ≥1 mM but displayed substantial inhibition of parasite growth suggesting alternate mechanism of action for these compounds. The best analogue 10 among the series displayed IC50 value of < 31 μM (Fig. 4).
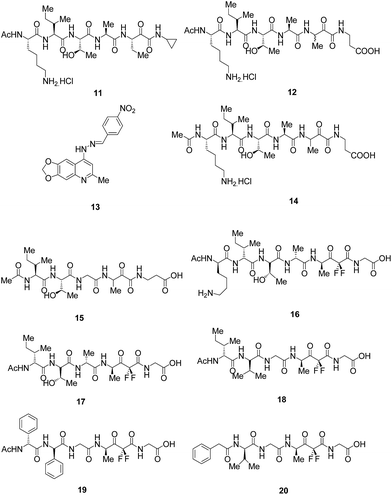
Molecular modelling approach was utilised along with existing understanding of SUB1 substrates and recombinant expression and characterization of additional PfSUB1 orthologues to investigate active site architecture and the substrate specificity of P. falciparum, and its orthologues from the two other major human malaria pathogens P. vivax and P. knowlesi, as well as from the rodent malaria species, P. berghei. The results disclosed the presence of the unusual features of SUB1 substrate binding cleft together with the requirement of interaction with both the prime and non-prime side residues of the substrate recognition motif thus supporting the observation obtained by crystallography studies. SUB1 catalysed the cleavage of conserved parasite substrates in all the parasites species which is well supported by the species-specific co-evolution of protease and substrates. To target PfSUB1, peptidyl alpha-ketomides (Fig. 5) based on authentic PfSUB1 substrate were designed and compound 11 completely inhibited the substrate hydrolysis, thus acting as fast-binding inhibitor of PfSUB1 with IC50 value of ∼6.0 μM and the IC50 values for rPvSUB1 and rPkSUB1 inhibition were ∼12 μM and ∼6.0 μM, respectively. The inhibitory potency was found to be increased by incorporating the carboxyl moiety to introduce prime side interactions with protease as exemplified by 12 possessing IC50 against PfSUB1 and PkSUB1 of ∼1.0 μM, and an IC50 against PvSUB1 of ∼2.0 μM. Thus, the development of these selective inhibitors based on PfSUB1 substrates which also exhibited inhibition of both rPvSUB1 and rPkSUB1, raises the prospect of developing broad-spectrum antimalarial agents targeting SUB1.22
Quinolylhydrazone, 13 was identified as hit compound for PfSUB1 (IC50 = 20 μM) through high-throughput screening of proprietary library of compounds. SAR studies of this set of compounds suggested the dependence of inhibitory activity on the hydrazone moiety attached to an aromatic ring possessing a strong electron withdrawing group. The mechanism of inhibition of the enzyme was reported to be either competitive or covalent reversible.24
Based on the previously reported hexapeptidic ketomide 14 inhibitor of PfSUB1,20,22 peptidic α-ketomides as PfSUB1 inhibitors were synthesised. SAR analysis was carried out to predict the crucial interactions that are beneficial for increasing the enzymatic activity. The SAR predictions revealed the dependence of inhibitory activity over serine trap (ketoamide) and on P1′–P1–P4 amino acids which determine the enzyme binding interactions. The studies clearly showed the preference for charged β-aminoalanine in the P1′ position while small size substituents like ethyl group is preferred in the P1 position. P2 position craves for glycine amino acid over alanine while threonine is required at P3 position to provide a solvent-exposed hydrophilic side chain to minimize solvation penalty. Isoleucine present at P4 position is desired to make crucial hydrophobic interactions for improved inhibitory activity. This optimization of side chain led to the pentapeptidic α-ketoamide 15 (Fig. 5) which is less polar than the parent hexapeptidic analogue 14 that exhibited IC50 of 0.9 μM similar to the compound 14. Thus, this compound can be explored further to develop as a lead against PfSUB1.25
In order to address the issue of low potency, lack of selectivity or poor cell permeability associated with reported inhibitors,22,24 rational drug-design approach was used for the synthesis of novel difluorostatone based inhibitors and evaluated them for inhibition of PfSUB1. The enzymatic assay led to the identification of compounds 16, 17 as potent PfSUB1 inhibitors (IC50 = 0.6 μM) possessing selectivity over mammalian proteases (Fig. 5). The homology model built for analysing the binding poses of the compounds were found to be in good agreement with crystal structure of PfSUB1. The docking results clearly revealed that both the potent compounds exhibits a series of interactions with the key residues present in the binding cleft of the enzyme. The enzyme/inhibitor interaction pattern proposed in the study will guide for the future optimization of this novel class of potent PfSUB1 inhibitors.20
SAR was further carried out on the previously identified inhibitors20 and reported novel and potent inhibitors of PfSUB1 based on rational drug designing approach. Compounds, 18 and 19 bearing non-natural amino acids (Fig. 5) were found to be most potent inhibitors reported to date with IC50 values of 0.25 and 0.28 μM respectively. Docking study carried out on the compound, 18 clearly fulfilled the key structural prerequisites outlined previously which are important for achieving inhibitory activity: presence of difluorostatone moiety, terminal carboxylic group at the P1′ site and three residues (P2–P4) at the non-prime side of the inhibitor. Furthermore, compound 20 with only two amino acid residues on the P-side, characterized by reduced peptidic character provide an efficient strategy for the development of inhibitors possessing decreased peptidic character.21
2. Food vacuole
P. falciparum digestive food vacuole plays a central role in the metabolism of the parasite where hemoglobin is degraded in the acidic environment to yield heme which is polymerized into insoluble hemozoin pigment and globin which is further degraded into individual amino acids.26 The whole process of globin digestion is carried out by various endo- and exo-peptidases in the specialized organelle, food vacuole.14 These constitute an important target since they are crucial for the survival of parasite.2.1.1. Aspartic proteases inhibitors. Plasmepsins (PMs) are pepsin like aspartic proteases found in P. falciparum which are involved in hemoglobin digestion occurring in digestive vacuole during the intra-erythrocytic phase of infection.28 The P. falciparum genome encodes ten PMs out of which PM I, PM II, PM III known as histo-aspartic protease (HAP) and PM IV reside in acidic vacuole and degrades hemoglobin whereas others present outside digestive vacuole supposed to play different roles.29 Various inhibitors have been reported in the literature and more potent are being developed with better pharmacokinetic profile.
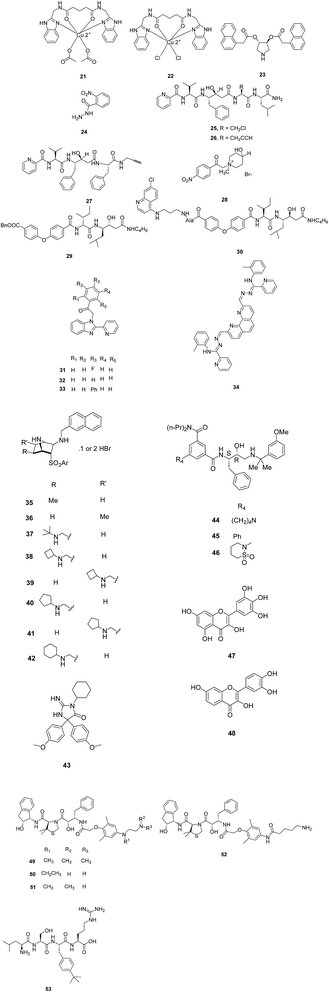
A new class of copper(II) nanohybrid solids 21 and 22 was reported (Fig. 7) and evaluated for their in vitro antimalarial potential against P. falciparum chloroquine-sensitive isolate (MRC 2). These nanohybrids exhibited activity with IC50 values of 0.032 μg ml−1 and 0.025 μg ml−1, respectively which is quite similar to standard drug chloroquine (IC50 = 0.020 μg ml−1). Moreover, Lineweaver–Burk plots for the inhibition of PM II by both the solids revealed the competitive inhibition with respect to the substrate. The selectivity over human cathepsin D (hCatD) and non-toxicity make them excellent leads to be developed as antimalarial compounds. Modelling experiments on PM II further established that both nanohybrid based compounds interacts with active site residue Asp214 which is part of the catalytic dyad. Benzimidazole group of 21 binds hydrophobically to the S1–S3 subsites.30
A series based on pyrrolidine scaffold, synthesised originally as HIV-1 protease inhibitors, was also evaluated for PM II and IV inhibition potential. Some of the inhibitors were found to be active in nanomolar range against both the isoforms. Compound 23 exhibited inhibition with Ki values of 0.10 and 0.09 μM against PM II and PM IV, respectively. Although the compounds were active in nanomolar range but the selective inhibition of PM over hCatD was not addressed for the most active compounds.31
Hydrazide and hydrazine derivatives were also identified as novel aspartic protease inhibitors through virtual screening of an in-house library followed by enzyme inhibition. This screening process identified five virtual hits which exhibited inhibition of both hCatD and P. falciparum PM II with IC50 in the low micromolar range of 1–2.5 μM but still a lot of work need to be done to discover lead molecules with high potency and selectivity towards PM II over hCatD.32
Four peptidomimetic analogues designed to act as mechanism based inhibitors of PM II were also evaluated. Three of these derivatives 25, 26 and 27 exhibited potent irreversible inhibition of the enzyme with IC50 values in the low nanomolar range (Fig. 7). Compounds 26 and 27 displayed antiparasitic activity with IC50 values of 7.7 and 9.2 μM while compound 25 was found to be poor inhibitor in growth assay suggesting the importance of activatable propargyl group in order to retain its antiparasitic activity. Although these compounds did not demonstrate reasonable selectivity of PM II over hCatD, optimization studies need to be carried out to increase their selectivity.33
Three nitrophenacyl derivatives of N-methyl-4-hydroxy piperidine were synthesized and evaluated for its enzyme inhibition potential. The compound 1-methyl-1-(4′-nitrophenacyl)-4-hydroxypiperidinium bromide with p-nitro group (28) was reported most active at the conc. of 1.0 μM with 22% inhibition in comparison to 100% inhibition by standard compound pepstatin.34 New potent inhibitors of P. falciparum CQ resistant W2 strain were developed by adopting dual drug strategy. 7-Chloro-4-aminoquinoline ring system was introduced in the statine double drugs in order to improve the accumulation of PM inhibitors inside the food vacuole. These compounds were tested against PfPM II, Pf, Po, Pv and PmPM IV and against D10 (CQ sensitive) and W2 (CQ resistant) P. falciparum strains. Although compound 29 showed potent inhibition against PfPM II, PfPM IV and PoPM IV but it showed moderate inhibition against P. falciparum strains. Based on enzymatic activity as well as growth inhibition assay, compound 30 with high selectivity over hCatD can be considered as potential antimalarial candidate.35
The selectivity towards PM II over hCatD was also improved by synthesizing novel benzimidazole derivatives. Enzyme inhibition data revealed that compounds 31 and 32 (Fig. 7) exhibited three times more selectivity towards PM II (IC50 = 10 μM) in comparison to hCatD. In the series, the compound 33 was found to be most potent and selective inhibitor of hCatD with IC50 = 2.0 μM and it exhibited highest antiparasitic activity with IC50 = 0.16 μM. The antiplasmodial data for this series laid emphasis on the importance of phenyl and a chlorine group at the para position.28
Non-peptide inhibitors of PM II from chemical databases of Specs and MayBridge were identified on the basis of structure-based virtual screening. Five novel non-peptide inhibitors with moderate potencies having IC50 in the range from 4.62 to 9.47 μM were identified. The lead compound, 34 displayed 68% inhibition of PM II with IC50 value of 4.62 μM.29 Several docking and molecular modelling studies on PM II showed interactions (H-bonds) with the important catalytic residues (Asp34 and Asp214) and also with other key residues such as flap residue Val78, residues Ser218 and Gly36 in close proximity to the catalytic dyad with compounds 25, 33 and 34.28,29,33
Structure based design of substituted 7-azanorbornenes was reported that was synthesised by hydroformylation reaction of substituted 7-azabicyclo[2.2.1]heptane core. The main concern behind this synthetic step was to introduce a new vector which could occupy S1′ pocket. The in vitro evaluation of these derivatives revealed high potency against three plasmepsins: PM I, II and IV in the nanomolar IC50 range and good selectivity over hCatD and E. The molecular modelling studies clearly depicted the importance of occupation of cycloalkylaminomethyl substituent at 6th position in S1′ pocket which increases the binding affinity and selectivity particularly in the case of PM IV.36
SAR analysis and optimization studies of aminohydantoins were performed to identify potent nanomolar range inhibitors of PM II and PM IV. The presence of cyclohexyl group on the N-3 position of aminohydantoin displayed improved antimalarial potential while inhibition of human aspartic proteases (BACE) was reduced. Thus, compound 43 can be further optimized for antimalarial drug development on the basis of low molecular weight, modest lipophilicity and oral bioavailability.37
Structurally simplified analogues 44 and 45 of potent hydroxyethylamine-based PM IV inhibitor 1SR (TCMDC-134674), 46 were identified in a GlaxoSmithKline cell based screening campaign. X-ray crystal structure of PM II bound with 46 shed light on the interaction between the hydroxyethylamine core of 46 and the PM II catalytic dyad Asp34–Asp214, thus provided structural insight for the design of potent and selective inhibitors (Fig. 6). SAR studies revealed that importance of R-configuration of the central hydroxyl-bearing stereo centre for plasmepsin inhibition. Further replacing 2-(1,2-thiazinane-1,1-dioxide) moiety by nonplanar hydrophobic groups such as piperidinyl and cyclohexyl resulted in increased potency against PM I and II due to the favorable interaction with Val78, while planar and more compact groups were responsible for PM IV inhibition, possibly, due to favorable flap-closing interactions, indicating selectivity for PM IV over CatD inhibition.38
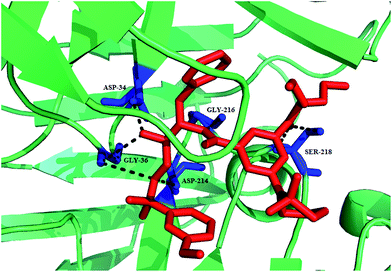 | ||
| Fig. 6 A ribbon diagram of PM II with inhibitor 46 shown in stick presentation (red). Active site residues (Asp34 and Asp214) and residues in vicinity to these (Ser218, Gly216 and Gly36) are depicted as blue sticks. The plausible hydrogen-bonding interactions are shown as black broken lines. The figure was made on PyMol using crystal structure coordinates of PM II bound to 46 (PDB ID: 4CKU). | ||
Inhibitory potential for some dietary flavonoids was investigated against P. falciparum FP-2 and PM II and identified two flavonol compounds, myricetin (47) and fisetin (48) as the dual inhibitors (Fig. 7). Compound 47 exhibited IC50 values of 1.5 and 7.2 μM while 48 displayed IC50 values of 4.9 and 7.8 μM against FP-2 and PM II, respectively. Kinetic investigation and molecular docking were carried out on these flavonoids to explore the inhibitory mechanisms which depicted that 47 and 48 are well docked in the active grooves of the enzymes through a number of hydrophobic as well as polar interactions. These dual inhibitors which act on multiple targets are supposed to possess synergistic activity thus prevent the emergence of drug resistance during malaria treatment.39
Plasmepsin inhibition potential was improved further by substituting 2-aminoethylamino groups to allophenylnorstatine-containing plasmepsin inhibitors that were identified previously40,41 and their in vitro antimalarial activity was investigated. On exploring SAR of the methyl or ethyl substituents on the amino groups, compounds 49 (KNI-10743) and 50 (KNI-10742) were found to be highly potent PM II inhibitors (Ki < 0.1 nM). These peptidomimetics reportedly exhibit antiplasmodial activity with EC50 of 0.36 and 0.46 μM, respectively. One of the peptidomimetics 51 (KNI-10740) was found to have highest antimalarial activity with EC50 of 0.19 μM. Binding model of compound 49 with PM II (PDB ID, 1SME) predicted a new hydrogen bonding interactions between 2-aminoethylamino groups with Asp130, present outside the enzyme active site, which may enhance PM II inhibitory potential.42
Inhibitor (KNI-10740) 51 was optimised to avoid drug resistance mechanism of multi-drug resistant TM91C235 strain of P. falciparum. The PM inhibitor, 51 exhibited 5-fold reduced activity against this resistant strain. A hypothesis was stated that reduction in inhibition potential is due to the structural similarity between 2-aminoethylamino substituent of 51 and chloroquine. To prove the hypothesis, moiety was modified which led to the identification of 52 (KNI-10823) that counters drug-resistant mechanism of TM91C235 strain.43 A series of short peptide based compounds was evaluated against D6 and W2 strains of P. falciparum which showed potent antiplasmodial activity with IC50 in the range 1.4–4.7 μg ml−1. The high potency of these peptides against drug resistant strain indicated their efficacy in the treatment of drug-resistant malaria parasites. The selected compounds were further evaluated for PM II inhibition and the most potent compound, 53 was found to have maximum inhibition of 25%. These were reported non-cytotoxic making them suitable antimalarial candidate (Fig. 7).44
2.1.2. Cysteine protease inhibitors. P. falciparum encodes four cysteine proteases from the papain family called falcipains (FPs): FP-1, FP-2, FP-2′, FP-3. Out of these, FP-2 and FP-3 have been identified as potential therapeutic targets where both contribute to degradation of host hemoglobin in the digestive vacuole.45 This proteolytic process generates amino acids which are utilized by erythrocytic malaria parasites for protein synthesis thus aids in parasite survival and development.31 FP-2 is also involved in erythrocyte rupture by the cleavage of cytoskeletal elements such as ankyrin and band protein 4.1, required for the stability of red blood cell membrane.46 Since hemoglobin digestion is crucial for the survival of malaria parasite, the inhibition of FP-2 and FP-3 is the promising way to effectively treat the disease.47 Several groups have characterized and validated FP-2 and FP-3 as potential drug targets so simultaneous inhibition of both FPs will be a powerful strategy to achieve parasite death. The FP inhibitors reported so far, have been grouped into different classes: peptide based, peptidomimetics and non-peptidic inhibitors depending on their core structural features.
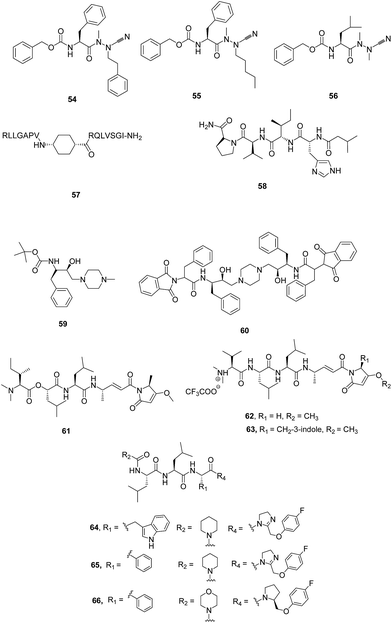
I. Peptide based inhibitors. Azadipeptide nitriles were reported as potent inhibitors of FP-2 and FP-3 as displayed by inhibition of parasite's hemoglobin-hydrolysing cysteine-proteases. These had also shown structure-dependent antimalarial effect against chloroquine-sensitive (3D7) and chloroquine-resistant (Dd2) lines of cultured P. falciparum malarial parasites. Compounds 54 and 55 (Fig. 8) both possessing phenylalanine in P2 and phenylethyl and pentyl in P1, respectively, exhibited the most potent activity against P. falciparum parasites, with IC50 values of ∼4.0 and ∼3.0 μM, respectively suggesting the importance of P1 substituents as well as azadipeptide nitrile scaffold for antimalarial activity. The compound 56, bearing a Leu side chain in the P2 position, was found to be potent inhibitor of FP-2 exhibiting an IC50 value of 59 nM. While compound 55 having phenylalanine at P2 and pentyl group at P1 displayed potent inhibitory activity with IC50 value of 110 nM against FP-3. These results suggested that there existed a correlation of enzyme inhibitory and antimalarial activity in this series of compounds but differences exist when comparing structure–activity relationship between isolated enzyme and parasite suggesting the synergistic action of a combination of hemoglobinases on the inhibition of parasite growth. This new class of antimalarial compounds provides greater structural variety into P1 position, which is quite feasible.48
Small peptides were synthesised mimicking the protein–protein interactions between FP-2 and chicken egg white cystatin (CEWC) which were designed based on the co-crystal structure of FP-2/CEWC. Three regions were identified in CEWC primary sequence through molecular modelling studies which made contacts with FP-2 active site: the N-terminal sequence (6RLLGAPV12), a β-hairpin loop (52RQLVSGI58) which is in close contact with FP-2 and another β-hairpin loop (103PW104) which is involved in minor interactions with FP-2. The peptide based mimics were designed taking into consideration these interacting motifs which contained different linkers: γ-aminobutyric acid (GABA), cis-4-aminocyclohexane carboxylic acid (cis-ACHC), a macrocycle formed by GABA and two cysteines joined by a disulfide linkage. The effect of the synthesized peptides was evaluated on FP-2 activity which revealed that peptides bearing the cis-ACHC were found to be more active than peptides containing GABA residue. Some of the compounds inhibited FP-2 in the micromolar range and produced morphological abnormalities in the food vacuole. Compound 57 emerged as most active compound with Ki value of 2.0 μM. Though the potency of these peptides are lower than cystatin, but the tendency to alter the food vacuole and the capacity to cross membranes may be beneficial in developing a new class of anti-malarial drugs.49
A new acyltetrapeptide, falcitidin, 58 was identified from methanolic extract from a myxobacterium Chitionophaga sp. Y23 which was isolated from soil collected in Singapore. Biological evaluation of this peptide against FP-2 showed IC50 value of 6.0 μM while it is ineffective against PM II (IC50 = 50 μM in FRET assay) and serine protease, tryptase (IC50 > 90 μM). Low yield and inconsistent production of the peptide led to the total synthesis of the 58 whose structure was confirmed as isovaleric acid-D-His-L-Ile-L-Val-L-Pro-NH2. 58 is different from other peptide-based inhibitors as it does not possess reactive groups like halomethyl ketones, vinyl sulfones, aldehydes, α-ketoamides and aziridines, which covalently bind to cysteine in the active site.50 In the search of more potent inhibitor of FP-3, Rathi et al. reported novel peptide based self-assembled nanostructures possessing hydroxylamine isostere. Spherical nanostructures 59 and 60 were screened against anti-malarial target, FP-3, involved as a major hemoglobinase of P. falciparum (Fig. 8). Hemoglobin hydrolysis assay data strongly indicated that 60 can completely inhibit FP-3 activity at an effective conc. of 1.5 μM as evidenced from its inability to act on hemoglobin, its natural substrate. Thus, bis-hydroxethylamine based peptide nanostructures exhibit excellent inhibitory activity against malarial cysteine proteases.47
Analogues of natural product gallinamide A, 61 were reported as potent inhibitors of falcipain cysteine proteases. Some of the analogues (62 and 63) exhibited potent inhibition of FP-2 and FP-3 as well as potent antiplasmodial activity strongly suggesting the presence of olefinic functional group within α,β-unsaturated imide moiety along with N-acylpyrrolinone unit for the potent inhibition. Some of the analogues with alike peptide backbone but with variation in the side chain of pyrrolinone unit and in the substitution of the enol of pyrrolinone exhibited equipotent inhibition as that of chloroquine against 3D7 strain and high potency against Dd2 parasite as well.51
Various analogues were synthesised based on the structure of pseudotripeptide designed using in silico screening tools and three dimensional structures of cysteine proteases like FP-2, FP-3 and papain. Out of 23 compounds, 8 compounds exhibited submicromolar inhibition of FP-2 with Ki values being in the range 0.2 μM to 0.8 μM while five compounds showed inhibitory activity against P. falciparum 3D7 cultures with IC50 less than 10 μM. Three compounds, 64, 65 and 66 selected on the basis of enzyme inhibition and antiparasitic activity were evaluated against Dd2 and MCamp (Malayan patient isolate with an unknown drug resistance profile) revealing compound 64, 65 to be the most potent inhibitor against both the isolates (Fig. 8).52
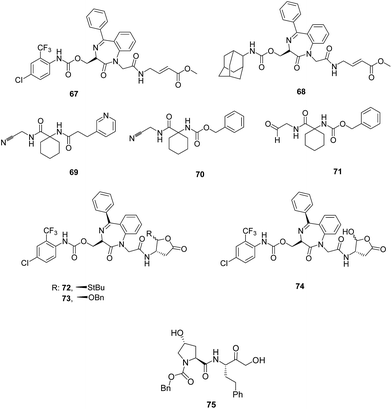
II. Peptidomimetics. Constrained peptidomimetics were reported based on benzodiazepine scaffold serving as mimic of fragment D-Ser-Gly and endowed with vinylester warhead. These derivatives were structurally related to previously reported irreversible inhibitor of FP-2, compound 67. Structural variations in the P1 site and substitution at the aromatic rings of benzodiazepine moiety were not a suitable design for constructing a favourable motif. Introduction of bulkier group, adamantyl at the side chain of serine at P3 site produced potent inhibitor, 68 (Fig. 9) with remarkable inhibition potential against FP-2 (Ki = 0.014 μM) and rhodesain (Ki = 0.0026 μM) as well as good selectivity over human cysteine proteases.53
Peptidomimetic nitriles as selective inhibitiors of FP-2 were synthesised based on the structure-based molecular modelling approach. The designing of these inhibitors were mainly focused on the optimal occupation of the selectivity-determining subpockets. The biological potential of this series of compounds containing nitrile as head group was assessed in order to gain insights into the binding site properties of the target enzyme. The compounds designed based on lead compound 69 exhibited covalent, reversible inhibition of the target enzyme with inhibitory affinities found in the nanomolar range (Ki = 17.2–2.1 μM). The carbamate analogue 70 showed Ki value of 1.2 μM. The most active compound, 71 (Fig. 9) bearing aldehyde group exhibited Ki value of 0.065 μM which may be due to high reactivity of this functionality as compared to the nitrile group but it also binds to both cathepsin B and L. Majority of the synthesized compounds displayed selectivity over human cathepsin B and L, and the serine protease α-chymotrypsin. Thus, the enzyme inhibition results clearly emphasised the optimal electrophilicity of the nitrile group as well as perfectly occupied selectivity-determining S2 pocket.54 Lipophilicity of 1,4-benzodiazepine scaffold was further improved by incorporating protected aspartyl aldehyde warhead which yielded the novel peptidomimetics, thioacylals and acylals. Trans diastereomers 72 and 73 (Fig. 9) exhibited potent enzyme inhibition in reversible manner with a Ki value of 8.2 μM and 8.9 μM, respectively almost equipotent to its parent compound 74 (Ki = 4.0 μM). The ability of both the derivatives to inhibit Plasmodium growth significantly increased as compared to the parent compound due to increased lipophilicity (IC50 = 28.5 and 49.5 μM). The compound 72 may also be developed as antiprotozoal agent as it emerged as a potent rhodesain inhibitor (Ki = 0.7 μM) and this also possessed potent trypanocidal activity with an IC50 value of 14.2 μM.55
Weldon et al. synthesised and reported a new series of peptidomimetic pseudo-prolyl-homophenylalanylketones and evaluated them for FP-2 and FP-3 inhibition as well as antiparasitic effect. Among the 22 compounds, 75 possessing homophenylalanine-based α-hydroxyketone linked Cbz-protected hydroxyproline emerged as the most potent inhibitor with IC50 values of 80 nM, 60 nM and 7.70 μM against FP-2, FP-3 and W2 strain of Plasmodium, respectively. In silico studies carried on these peptidomimetics shed light on important protein–ligand structural insights and role of water molecules in the active sites of these protease isoforms by utilizing WaterMap study. Thus, the pseudo-dipeptide and its analogues offered an interesting aspect to be carry forward for the design of competitive inhibitors of falcipains for antimalarial therapy.56
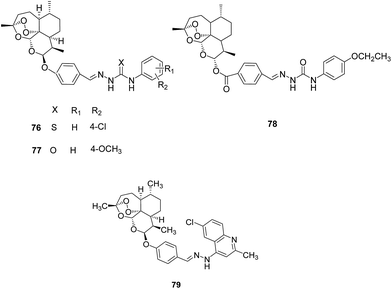
III. Artemisinin derivatives. A series of 10-substituted artemisinin derivatives was synthesised by incorporating (thio)semicarbazone and evaluated them for FP-2 inhibition potential (Fig. 10). Most of the compounds displayed potent inhibition in low micromolar range and compounds 76 and 77 incorporating thiosemicarbazone and semicarbazone, respectively, were found to be the most effective with IC50 value of 0.46 μM. Molecular docking studies performed with the most active inhibitor, 76 predicted the plausible binding mode as well as binding affinity. It is involved in the hydrogen bonding interactions with Gly83, Leu172 and Asp234.57
Another series of dihydroartemisinin derivatives was developed based on (thio)semicarbazone scaffold and evaluated them against FP-2 enzyme. The enzyme assay showed that most of the synthesised compounds displayed significant inhibition with IC50 values in the range of 0.29–10.63 μM and 78 presented IC50 value of 0.29 μM against FP-2, the most potent among them, formed hydrogen bonds with Cys42 and Gly83 as predicted by molecular docking studies.58
Further a series comprising 4-quinolylhydrazone and artemisinin cores was synthesised and evaluated for FP-2 inhibitory activity. The enzymatic assay revealed that all the compounds exhibited excellent potential against recombinant FP-2 (IC50 = 0.15–2.28 μM). The best inhibitor 79 of this series exhibited IC50 value of 0.15 μM. Molecular modelling study further indicated formation of hydrogen bonds as well as hydrophobic contacts with key residues of the receptor pocket.59
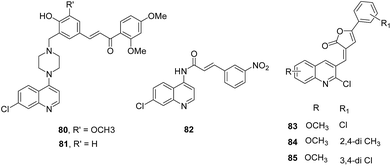
IV. Quinoline based analogues. Guantai et al. designed and synthesized enone- and chalcone-chloroquinoline hybrid analogues based on the previously reported promising antimalarial leads (Fig. 11).60 These analogues were developed with the aim to improve solubility and antimalarial potential. In silico predictions provided favourable results in terms of improved solubility at low pH as well as permeability properties but indicated susceptibility to hepatic metabolism which was very well supported by in vitro results. Compounds 80 and 81 were reported as the most potent leads with the mechanism predicted to be FP-2 inhibition. But the primary mechanism underlying the potency of other compounds was inhibition of hemozoin formation as suggested by biochemical studies.61
Novel cinnamic acid/4-aminoquinoline conjugates linked through a retro-enantio dipeptide were developed as dual action drugs. These derivatives (with or without spacer) were assessed for their ability to inhibit hemozoin growth, FP-2/3 inhibition as well as blood stage P. falciparum growth. The results did not exhibit correlation between blood stage growth and enzyme inhibition as hybrids with dipeptide were active against blood stage P. falciparum and hemozoin growth while compounds lacking spacer were found to be better FP-2 inhibitors, thus suggesting different mechanism of action. One of the compounds, 82 was found to be the best inhibitor with IC50 value of 14.2 μM. The results clearly indicated that this dipeptide plays a critical role in inhibiting P. falciparum growth and hemozoin formation and this is more preferable to its counterparts with L-amino acids.62
Quinoline-substituted furanone derivatives were synthesised and evaluated for their antimalarial potential. The compounds 83, 84 and 85 displayed excellent antiparasitic activity with IC50 in the range of 0.50–0.72 μg ml−1. A preliminary SAR emphasised on the presence of electropositive character for enhancing antimalarial activity. The FP-2 enzymatic assay on these three selected compounds suggested that 83 and 84 exhibited 58% and 62% inhibition of FP-2, respectively and could be developed as selective FP-2 inhibitors.63
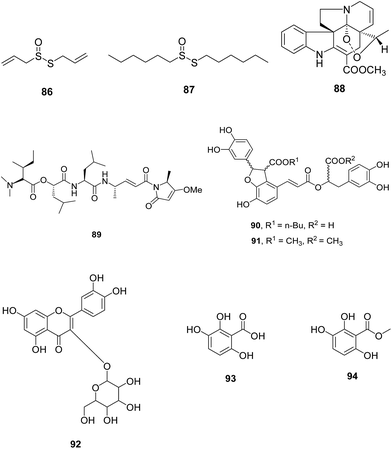
V. Natural product based analogues. Allicin, 86 main biologically active component of garlic extracts, and its derivative 87 having large lipophilic groups were identified as inhibitor of cysteine proteases FP-2 (Fig. 12). These compounds inhibited FP-2 and other cysteine proteases rhodesain, cathepsin B and L in low nanomolar range. These derivatives displayed potent antiplasmodial and antitrypanosomal activities in micromolar range. The SAR study emphasized on the presence of primary carbon atom in vicinity to the thiosulphinate sulphur atom for improved enzyme activity. Since compound 87 bearing dihexyl group is more stable in solution, so it can be taken further as lead molecule for designing allicin derivatives with enhanced affinities.64
Wang et al. isolated catharoseumine, 88 a monoterpenoid indole alkaloid having a peroxy bridge moiety from the whole plant of Catharanthus roseus. It exhibited potential inhibition of FP-2 with IC50 value of 4.06 μM.65 Stolze et al. synthesized some analogues of natural product symplostatin 4 (Sym4), 89 reported to be potent nanomolar growth inhibitor of malaria parasite P. falciparum with EC50 = 0.7 μM. The effect of 89 was seen in the form of red food vacuole defect which indicated the blockage of initial stages of hemoglobin degradation. This effect was found due to FP-2, 2′ and 3 inhibition at low nanomolar conc. (1.5 nM). Sym4 exhibited different potencies towards these cysteine proteases as FP-2/2′ were inhibited more potently than FP-1 and FP-3. The critical importance of unusual methylmethoxypyrrolinone (mmp) group of Sym4 was established by the low potency of synthesised analogues of Sym4 which suggested that the compounds bearing mmp motif can be considered for the development of antimalarial FP inhibitors (Fig. 12).66
A fraction was isolated from the Caribbean coral Plexaura homomalla that was enriched in tight-binding protease inhibitors and evaluated for its antiparasitic effect against P. falciparum and T. cruzi. The inhibitor-enriched fraction displayed Ki values of 1.99 nM and 4.81 nM for FP-2 and cruzipain from P. falciparum and T. cruzi, respectively. The antiparasitic results showed reduced growth of chloroquine-resistant P. falciparum strain Dd2 with IC50 value of 0.46 μM. This fraction also resulted in the reduction of Vero cells infection by T. cruzi trypomastigotes at sub-micromolar conc.67
Ten natural compounds were identified as FP-2 inhibitors through structure-based virtual screening of an in-house Natural Product Database (NPD) that exhibited good potencies against FP-2 with IC50 in the range of 3.18 to 68.19 μM. Among them, compounds 90 and 91 exhibited promising potential with IC50 values of 3.18 and 3.77 μM, respectively. Some of the inhibitors were evaluated for their antiparasitic effect and compound 92 inhibit chloroquine sensitive (3D7) and resistant strain (Dd2) in low nanomolar range (IC50 = 5.54 μM and 4.05 μM against 3D7 and Dd2, respectively). Compound 90 was further assessed to have various hydrogen bonds and hydrophobic interactions with active site residues.68
Two compounds 2,3,6-trihydroxy benzoic acid (93) and 2,3,6-trihydroxy methyl benzoate (94) were extracted based on activity-guided fractionation from the air-dried fruits of Sorindeia juglandifolia. The collected fractions were tested for inhibition potential against P. falciparum W2 strain and FP-2. Nine fractions showed inhibitory activity with IC50 value in the range 2.3–11.6 μg ml−1 for W2 and 1.1–21.9 μg ml−1 for FP-2. These were also active against the field isolates of P. falciparum. Purified compounds 93 and 94 (Fig. 12) also displayed potent inhibition against W2 (IC50 = 16.5 μM and 13 μM) and FP-2 (IC50 = 35.4 and 6.1 μM). In vivo assessment of compound 93 showed activity as well as safety in rodent malaria model thus can be further investigated for the development of antimalarial candidate.69
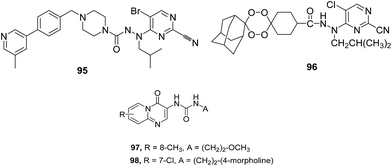
VI. Pyrimidine based analogues. Coterón et al. reported various heteroarylnitrile derivatives, among them, 5-substituted-2-cyanopyrimidine emerged as most promising series (Fig. 13). Sequential optimization of this series, considering different positions present in the parent scaffold, led to the identification of potent FP-2 and FP-3 inhibitors with nanomolar and subnanomolar range inhibition. Identified inhibitors exhibited activity in whole cell assay in micromolar range. Introduction of different substituents bearing amines at P3 position in the structure of lead compounds led to the improved enzymatic activities in the picomolar to low nanomolar range with antiparasitic activity in the low nanomolar range.45
To further support the observation that pyrimidine nitrile motifs could accommodate a variety of substituents at P3 position without significantly affecting enzyme inhibition, tetraoxane–pyrimidine nitriles with peroxide moiety at P3 position were developed (Fig. 13). These derivatives were evaluated against blood and liver stages of malaria as well as FP-2 enzyme. All the derivatives exhibited potent nanomolar activity against the three strains of P. falciparum and FP-2 in comparison to their vinyl sulphone counterpart. The most potent compound 96 displayed excellent FP-2 inhibition with IC50 = 3.4 nM as well as low cytotoxicity and good activity in the liver stage assay in vivo. Nitriles inhibit cysteine proteases by forming hydrogen bonds with catalytic residues, Cys42 and Gln36. Additional interactions were detected with Gly83 and Asn81.70 Pyrido[1,2-a]pyrimidin-4-ones were evaluated for FP-2 inhibition potential which displayed fairly good activity. The excellent FP-2 inhibition by compounds 97 and 98 with IC50 values of 6.0 and 7.0 μM, respectively emphasized the presence of urea moiety to enable the compounds to be potent FP-2 inhibitor (Fig. 13).71
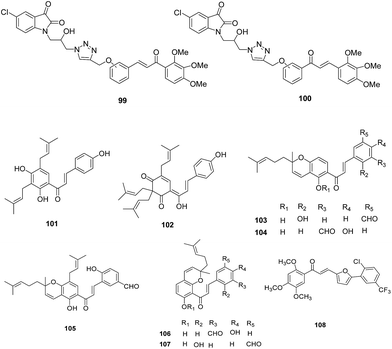
VII. Chalcone based analogues. A series of β-amino alcohol thiolactone–chalcone and isatin–chalcone hybrids was developed and evaluated for growth inhibitory activity against W2 chloroquine-resistant strain of P. falciparum and its inhibitory activity against recombinant FP-2. The results for a 36-member β-amino alcohol triazole library showed that the thiolactone–chalcones displayed promising antiplasmodial activity, with IC50 values ranging from 0.68 to 6.08 μM, than the isatin–chalcones with IC50 of 14.9 μM or less. While isatin–chalcones hybrids exhibited FP-2 inhibitory activity with meta-substitution preferred compound, thiolactone–chalcones lacked FP-2 inhibitory activity suggesting that these compounds act via impairment of other parasite pathways or targets.72
A library of 88 chalcones was synthesised as analogues of natural antimalarial agents, medicagenin, 101 and munchiwarin, 102. These derivatives were evaluated for their in vitro antimalarial potential, and compounds 103–107 were found with good antiparasitic activity, low cytotoxicity and good selectivity index (Fig. 14). In vivo results demonstrated rapid parasite clearance (71–98%) with increased survival time. The inhibitory potential of these compounds against FP-2 showed that the compound 107 inhibited FP-2 with IC50 of 4.6 μM. Docking study of chalcone based inhibitor, 107 with FP-2 showed formation of hydrogen bonds with S1′ and S1 subsites (Tyr206 Gln36, Cys39, Cys42 and His174). Alkenyl group of 107 formed hydrophobic contacts with Leu84 (S2) and Ile 85 (S3).73
An in-house library consisting of (E)-chalcones, (E)-N′-benzylidene-benzohydrazides and alkylesters of gallic acid against FP-2 was developed. Among these, (E)-chalcones were found to possess lower IC50 values with compound 108 emerged as potent FP-2 inhibitor displaying non-competitive inhibition with IC50 value as 4.9 μM.74
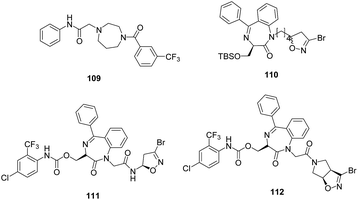
VIII. 1,4-Diazepan based analogues. A new class of FP-2 inhibitors was designed and synthesised using ligand based approach. The 2-(4-(substituted benzoyl)-1,4-diazepan-1-yl)-N-phenylacetamide derivatives were evaluated for FP-2 inhibition. Out of all the compounds, five compounds displayed >60% inhibition at 10 μM conc. while 109 was found to be most potent compound with 72% inhibition at the same conc. and forms several hydrophobic and hydrogen-bond interactions with active site residues as indicated by modelling studies.75
Novel antiprotozoal agents containing 1,4-benzodiazepine scaffold were developed through an optimization study of previously reported inhibitors.76 The novel compounds were synthesized by modifying or even removing the side chain attached at C3 position of the benzodiazepine scaffold. The nature and length of the spacer connecting the benzodiazepine ring and 3-bromoisoxazoline moiety were also considered. These derivatives were evaluated against rhodesain and FP-2 which displayed single-digit micromolar or sub-micromolar activity against one or both enzymes. Among these, the most potent FP-2 inhibitor was found to be 110 with Ki value 0.23 μM even more potent than the model compounds 111 and 112 exhibiting Ki value of 5.48 and 6.0 μM, respectively (Fig. 15).47
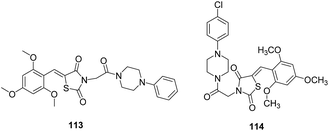
IX. Thiazolidine-2,4-diones. A series of 2,4-thiazolidinediones based on TZD 4 (thiazolidine-2,4-dione derivative), 113 was identified through structure-based virtual screening (Fig. 16). The compounds were synthesised and evaluated for their antimalarial potential which displayed low micromolar antiplasmodial activities against P. falciparum drug resistant W2 strain. FP-2 inhibition data revealed that compound 114 (IC50 = 11.23 μM) showed enhanced inhibitory activity in comparison to 113 (IC50 = 25.5 μM). In spite of significant antiparasitic activity, the most active compounds showed poor microsomal metabolic stability in the presence of human, rat and mouse hepatic microsomes. Molecular docking between FP-2 and 114 showed that chlorophenyl and the trimethoxyphenyl group occupied S2 and S1 subsites, respectively. The thiazolidine-2,4-dione scaffold was close to the active site of enzyme. His174 was shown to be in dipole–dipole interaction with the oxygen of inhibitor.77
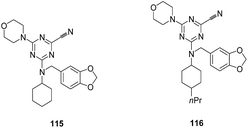
X. Triazine nitriles. Selective inhibitors of FP-2 and rhodesain from P. falciparum and T. brucei were identified through structure based drug design. FP-2 inhibition studies demonstrated preference for morpholine group in place of cyclopropylamine derivative or 2-methoxyethylamine groups in the S1 pocket and smaller size substituents were desired in the S2 pocket of the enzyme and 1,3-benzodioxol-5-yl and cyclohexyl substituents were preferred for S3 pocket. Compound 115, with an unsubstituted cyclohexyl moiety and morpholine group with 1,3-benzodioxol-5-yl group in the S2 pocket of the enzyme was found to be more potent against FP-2 (Ki = 20 nM) and it showed Ki = 8.0 nM against rhodesain (Fig. 17). Another, lead compound 116 exhibited highest affinity against the rhodesain enzyme with Ki values of 2.0 nM. Some of the compounds showed affinity for human cathepsin L in the low nanomolar range while selectivity was observed against human cathepsin B and the viral cysteine proteases and α-chymotrypsin. Despite their excellent enzymatic activity and good selectivity, compounds displayed moderate antiplasmodial activity and marginal activity against T. brucei rhodesiense, thus studies are required to improve its inhibition potential in the cell-based assays.78
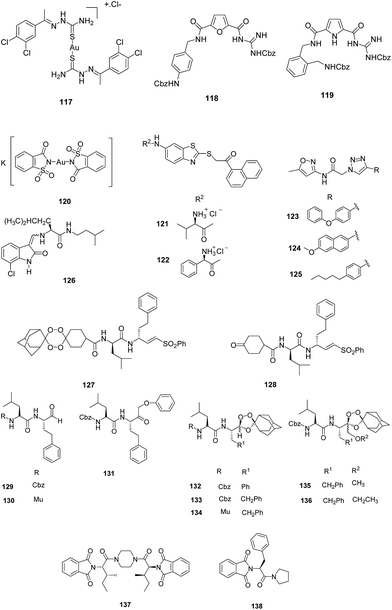
XI. Miscellaneous. Khanye et al. studied the inhibitory effect of gold(I) thiosemicarbazone complexes on FP-2 enzyme. The antiparasitic activity as well as enzyme inhibition revealed that coordination of gold(I) to thiosemicarbazone ligand enhanced their efficacy against P. falciparum as well as FP-2. The most potent inhibitor was gold(I) complex 117 with IC50 value of 0.87 μM. The improved activity of thiosemicarbazone gold(I) complexes may be due to the geometry adopted by gold(I) by holding thiosemicarbazones that allows favourable interactions of thiosemicarbazones with the target enzyme. Although no correlation existed between antiplasmodial activity and enzyme inhibition which suggests that the antiplasmodial activity of these complexes may be due to the inhibition of more than one target.79
Langolf et al. developed a new class of potent and reversible cysteine protease inhibitors based on N-protected-guanidino-furan and -pyrrole motifs (Fig. 18). The most potent inhibitor, 118 exhibited IC50 = 1.06 μM, while other active compounds showed IC50 in the range of 1.38–8.09 μM against P. falciparum. The excellent antiparasitic activity clearly emphasizes on the presence of guanidine moiety and the nonpolar aromatic part of the aniline building block. The enzymatic inhibition data revealed that compound 119 showed potent activity with Ki = 0.52 μM against FP-2. These derivatives also showed excellent activity against African trypanosomiasis and rhodesain. Moreover these compounds exhibited low or moderate cytotoxicity against mammalian cells.80
Micale et al. evaluated some structurally varied gold complexes for the FP-2 inhibition as well as for P. falciparum growth inhibition. The results obtained from both inhibition studies failed to establish any correlation between the two, thus supporting the findings by Khanye et al.79 Out of these gold complexes, K[Au(Sac)2], 120 displayed 66% inhibition of FP-2 which might be due to binding of Au(I) to the catalytic active site cysteine while auranofin was found to be potent inhibitor in growth assay with IC50 value of 0.142 μM.81
Shah et al. designed and synthesised combinatorial library comprising benzothiazole and triazole scaffolds based on core-hopping strategy. These compounds were evaluated for their inhibition potential of FP-2 which revealed fifteen analogues of both series with moderate inhibition of FP-2. Among these, two compounds, 121 and 122 with protonated amino groups from the benzthiazole series were found to inhibit both the enzymes: FP-2 and FP-3 which was well correlated with the results obtained by docking studies. Compounds possessing bulkier substituents, 123, 124 and 125 from the triazole series afforded moderate inhibition of FP-2 while none of the compounds, except 123 from the triazole series inhibited FP-3 (Fig. 18). Compound 121 also exhibited potency against chloroquine-resistant cultured P. falciparum with IC50 value in the lower micromolar range. Both these compounds, 121 and 122 also inhibited mammalian cysteine proteases of papain family suggesting that the polar residues present in the S2 pocket may not be critical to the selective inhibition of falcipain.82
Kumar et al. designed and synthesized 3-methylene-substituted indolinones and evaluated them as FP inhibitors and antiplasmodial agents. The enzymatic inhibition results revealed that indolinones containing a Leu-isoamyl recognition moiety were having moderate inhibition potential against FP-2 while none was active against FP-3. These compounds exhibited antiplasmodial activity in the low micromolar range against the chloroquine-resistant W2 strain of P. falciparum. Coupling of 7-chloroquinoline to 3-methylene-substituted indolinone resulted into potent antiplasmodial agents with no effect on FP-2 inhibition suggesting a different mode of action for these compounds. Thus, 3-methylene-indolinone-2-ones being potent lead compounds can be further developed into new antimalarial therapeutics. Molecular docking studies of 126 with FP-2 showed interactions with Cys42.83
Oliveira et al. designed and synthesised a novel class of tetraoxane–vinyl sulfone based hybrid compounds possessing high potency against chloroquine-sensitive and chloroquine-resistant P. falciparum strains in the low nanomolar range. Although these demonstrate weak to moderate inhibition of FP-2 with IC50 values ranging from 1.0 to 10 μM but the ability of efficient delivery of vinyl sulfones (FP-2 inhibitor) in the presence of iron(II) bromide in the parasite digestive vacuole makes them promising candidate with nanomolar protease inhibitory activity. Mechanistic aspect of these compounds were studied with 127 which indicated the presence of intact parent vinyl sulfone, 128 in the infected erythrocytes even after 48 h of post treatment with the hybrid compounds suggesting its long lasting effect. These results clearly indicated that masking of tetraoxane partner offers efficient strategy for diminishing the off-target effects of known drugs (Fig. 18).84
Gibbons et al. extended their combination chemotherapy approach through a single prodrug entity utilizing 1,2,4-trioxolane as protease inhibitor carbonyl masking group. They synthesized carbonyl based protease inhibitors and their endoperoxide hybrids. These were evaluated for recombinant FP-2 and FP-3 inhibition and antimalarial activity against 3D7 P. falciparum strain. Aldehydes 129 and 130 displayed potent and selective inhibition of FP-2 in low nanomolar range while only α-phenoxy analogue, compound 131 among the keto series inhibited both the FP-2 and FP-3 with low nanomolar activity. 129 and 130 also displayed low nanomolar activity against 3D7 P. falciparum strain. Among the endoperoxides hybrids, aldehyde prodrugs, 132, 133, 134 and ketone prodrugs, 135 and 136 were the most potent where 133 and 134 exhibited IC50 values of 55 and 35 nM against 3D7 strain (Fig. 18). Although there is more than 400 times difference in the FP-2 inhibitory potency between aldehyde 129 and endoperoxide 133, but similar activity against cultured parasites could be explained due to contribution from the activation and inhibitor release. Mechanistic approach was studied through ferrous mediated decomposition of these molecules which suggested that these compounds degrade to form carbonyl inhibitor along with C-radical formation.85
Singh et al. reported a new class of phthalimides functionalized with cyclic amines as antiparasitic agent and FP-2 inhibitor. Of all the synthesized derivatives, compound 137, bearing piperazine linker was found to be the most potent antimalarial agent with IC50 values of 5.97, 2.0 and 1.1 μM on incubation period of 42, 60 and 90 h, respectively, thus effectiveness is increased with increase in exposure time of the parasite to the compound. The compound 137 was also found to be effective against chloroquine resistant strain (Pf7GB) with IC50 value of 7.21 μM. The enzymatic assay revealed that compound 138 was the most active compound against FP-2 thus suggesting that phenylalanine residue play a critical role in FP-2 inhibition.86
2.1.3. Metalloaminopeptidases (MAPs). The neutral aminopeptidases M1 alanyl aminopeptidase (PfA-M1), M17 leucine aminopeptidase (PfA-M17) and M18 aminopetidase play an essential role in the growth and propagation of Plasmodium as these act during terminal stages of hemoglobin digestion by degrading small peptide fragments into free amino acids during the symptomatic erythrocytic stage of infection.87 Another study has revealed that PfA-M17 is associated with some other role in addition to hemoglobin digestion and found to be essential in the early life cycle of the parasite before the beginning of hemoglobin digestion. These are metalloenzymes which bear distinct arrangement of Zn2+ ion in their active site, therefore chelation of the metal ion in either enzyme inhibits its activity. Hence, those inhibitors which can effectively bind the metal ions will prove as a boon in malaria eradication campaign.88 Several groups have synthesized inhibitors incorporating metal binding scaffolds which have proven their potency by reducing the enzyme activity thus affecting parasite growth.
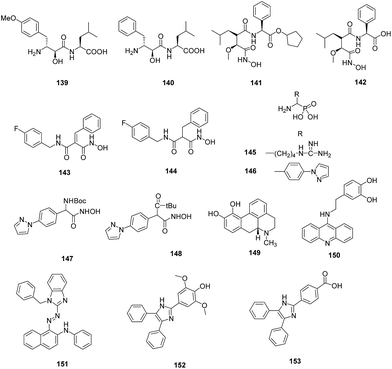
A diverse library of bestatin derivatives was synthesised that varied at the side chain of either the α-hydroxy-β amino acid (P1) or the adjacent natural α-amino acid (P1′) (Fig. 20). Screening of the library against PfA-M1 showed the Tyr(OMe) derivative (139) was the most potent inhibitor (Ki = 43 nM) suggesting that S1 pocket could accommodate large aromatic side chains as well. The derivatives were evaluated against P. falciparum which showed moderate potency with IC50 values in the micromolar range. The most potent inhibitor 139 exhibited IC50 of 6.4 μM which was slightly less potent than bestatin, 140 (IC50 = 3.2 μM) might be due to lowered permeability because of methoxy group.89
The antimalarial potential of CHR-2863, 141 and its acid analogue CHR-6768 (142) was evaluated. Enzymatic assay against PfA-M1 and PfA-M17 demonstrated that 141 was almost two-fold more potent (Ki = 1.4 μM) than its acid derivative (Ki = 2.4 μM) while found to be equipotent as 140 (Ki = 1.6 μM) against PfA-M1 enzyme. Both the ester and acid were found to be more potent (20 to 100 fold) against PfA-M17 than PfA-M1 while found to be 8-fold and 24-fold more potent, respectively against rPfA-M17 than bestatin. The acid, 142 (Ki = 0.025 μM) displayed three times greater inhibition constant than 141 (Ki = 0.075 μM). The in vitro antiparasitic evaluation demonstrated that ester (IC50 = 370 nM) exhibited 5-fold greater potency than the acid (IC50 = 2.0 μM) while bestatin showed IC50 value of 5.0 μM. Analysis of parasite morphology after treatment with 141 showed that it induces vacuolization in P. falciparum parasites similar to 140. Treatment of mice infected with murine malaria species P. chabaudi with 141 at the oral dose of 25 mg kg−1 daily reduced the peak parasitemia in all groups compared to the controls, with increased doses showed enhanced effect on parasite growth.90
SAR of a selective family of hydroxamate PfA-M1 inhibitors based on malonic scaffold was evaluated. Introduction of steric constraints at the malonic position led to the identification of 143 which exhibited nanomolar selective inhibition of PfA-M1 with IC50 value of 6.0 nM. It showed good physicochemical, in vitro plasma stability and potent in vitro antiplasmodial activity. “Fluoro-scanning” performed around hit molecule 143 and 144 identified para position on benzylamido group as the key position of halogen for activity (Fig. 20). The compound 143 was stable in microsomes, in plasma. The in vivo distribution study revealed that compound 143 reaches its site of action. Nonetheless, it was unable to kill the parasite at the conc. needed for enzyme inhibition. Thus, the results can be concluded that pharmacological activity of an inhibitor should not only be dependent upon its binding to the target molecule and its inhibitory potency but its ability to reach the target at an enough conc. for ample period of time. Docking study of compound 143 with PfA-M1 showed that S1 pocket undergo major conformational change on inhibitor binding and it can accommodate larger groups by making hydrophobic interactions thus supported the observation made by Velmourogane et al.89,91
Synthesis and structure–activity relationship of a small library of metal-chelating phosphonic acid arginine mimetics were reported that probe the S1 pocket of both enzymes. Evaluation of this library as inhibitors of the PfA-M1 and PfA-M17 aminopeptidases of P. falciparum found 1-amino-5-guanidinopentylphosphonic acid (145) potent against PfA-M1 with Ki value of 11 μM while rigidification due to introduction of phenyl group or reduction in the length of carbon chain reported detrimental to inhibition potential. Guanidino groups played a very important role in retaining the inhibitory activity. The compound (4-(pyrazol-1-yl)phenyl) (amino) methylphosphonic acid (146) emerged as most potent inhibitor of PfA-M17 with Ki value of 11 nM. It was also found to be modest inhibitor of PfA-M1 with Ki value of 104 μM involved in few interactions with the large S1 pocket of the enzyme, thus providing additional advantage to be developed as dual PfA-M1/M17 inhibitor. Further optimization of compound 146 in terms of increasing PfA-M1 inhibition potential and its physicochemical properties for antiparasitic activity is desirable to develop it as lead antimalarial candidate. X-ray crystal structures of lead inhibitors bound to these enzymes showed essential interactions within the active sites and shed light on the structural modifications required for making them dual inhibitor. Crystal structures of 145 and 146 with the PfA-M1 and PfA-M17, respectively were reported. In the PfA-M1-145 enzyme complex, N2 of guanidino group makes interactions with backbone oxygen of Glu572, a critical residue required for the formation of S1 pocket of PfA-M1. In the PfA-M17-146 complex, phosphate group of the inhibitor is involved in extensive interactions with the two metal ions and carbonate ion present in the active site of PfA-M17. The amine group of the inhibitor is involved in hydrogen bonding interactions with Asp379 and 399. The 4-(1H-pyrazol-1-yl)phenyl group fitted efficiently in the hydrophobic S1 pocket by forming hydrophobic packing interactions (Fig. 19).88
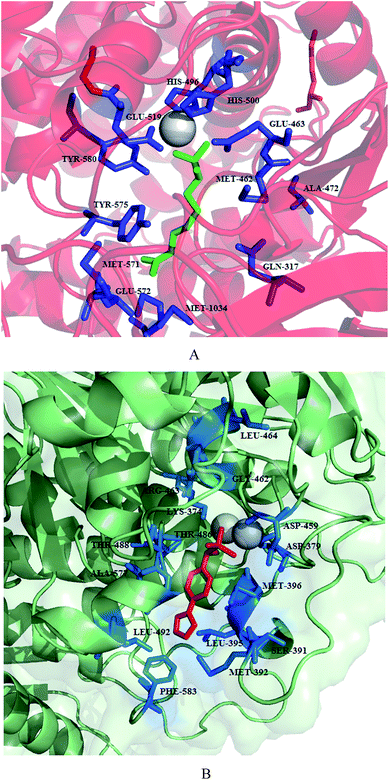 | ||
| Fig. 19 Crystal structure of inhibitors bound to the active site of metalloaminopeptidases. Carbon atoms of PfA-M1 are colored in red (A) and carbon atoms of PfA-M17 are colored in green (B). Zinc is shown as grey sphere in PfA-M1/PfA-M17. Inhibitors, 145 and 146 are colored in green and red, respectively. Figures were made in PyMol using PDB ID: 4K5L (PfA-M1) and 4K3N (PfA-M17). | ||
To improve the membrane permeability and plasma safety profile, Mistry et al. replaced the phoshonic acid group with hydroxamic acid-based compounds as zinc binding groups. This approach led to the identification of 147 as potent dual inhibitor of PfA-M1 (Ki = 0.8 μM) and PfA-M17 (Ki = 0.03 μM). This inhibitor was found to be 100-fold active against PfA-M1 than that of 145 while PfA-M17 potency was comparable to 145. This compound exhibited selective inhibition of PfA-M1 and PfA-M17 over PfM18AAP, another P. falciparum MAP. Crystal structure of both the enzyme with compound 147 revealed the reason behind improved potency that along with strong coordination to the zinc ion as well as probing the hydrophobic S1 pocket, N-Boc group was involved in binding within the S1′ pocket. SAR of this molecule by incorporating N-acyl group in place of N-boc group identified compound 148 exhibiting Ki = 0.7 μM against PfA-M1 comparable to that of compound 147 while same did not exist in case of PfA-M17. Replacement of N-boc group by N-fluorobenzoyl group helped in regaining potency against PfA-M17 but were unable to inhibit PfA-M1. Such modification can be explored further for potent PfA-M17 inhibition although it is unfavorable for PfA-M1 inhibition. The compounds were further evaluated for their antiparasitic effect and compound 148 with IC50 of 227 nM emerged as potent inhibitor against 3D7 malaria parasites. Though compound 147 exhibited comparable enzyme inhibition potential, IC50 value of 783 nM suggested the less capability of cellular penetration of N-Boc group than the corrosponding N-acyl derivatives. Lack of toxicity suggested that this series has potential to be developed as potent MAP inhibitors.87
A Molecular Libraries Probe Production Centers Network collection comprising ∼292![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds was screened to identify potent and selective PfM18AAP inhibitors. This was done by developing a fluorescence enzymatic assay using recombinant PfM18AAP enzyme and a fluorogenic peptide substrate (H-Glu-NHMec). This was further counter screened to identify compounds with nonspecific activity by cathepsin L1 (CTSL1) enzyme based assay. This high-throughput screening identified two structurally related compounds, CID 6852389, 149 and CID 23724194, 150 which exhibited micromolar potency and were inactive in CTSL1 titration experiments with IC50 > 59.6 μM (Fig. 20). Both compounds displayed non-competitive inhibition in micromolar range in PfM18AAP enzyme assay with Ki values of 3.39 and 1.35 μM. The compounds were found active in whole cell assay with IC50 values of 4.0 μM and 1.3 μM, respectively. Thus, these compounds will provide the basis for the identification of small molecule PfM18AAP inhibitors.92
000 compounds was screened to identify potent and selective PfM18AAP inhibitors. This was done by developing a fluorescence enzymatic assay using recombinant PfM18AAP enzyme and a fluorogenic peptide substrate (H-Glu-NHMec). This was further counter screened to identify compounds with nonspecific activity by cathepsin L1 (CTSL1) enzyme based assay. This high-throughput screening identified two structurally related compounds, CID 6852389, 149 and CID 23724194, 150 which exhibited micromolar potency and were inactive in CTSL1 titration experiments with IC50 > 59.6 μM (Fig. 20). Both compounds displayed non-competitive inhibition in micromolar range in PfM18AAP enzyme assay with Ki values of 3.39 and 1.35 μM. The compounds were found active in whole cell assay with IC50 values of 4.0 μM and 1.3 μM, respectively. Thus, these compounds will provide the basis for the identification of small molecule PfM18AAP inhibitors.92
A highly sensitive multiplex assay was developed for the rapid primary screening of “MMV 400” compounds against all the three metalloaminopeptidases of P. falciparum: PfA-M1, PfA-M17 and PfM18AAP. The screening showed that the selected compounds were ineffective in inhibiting PfM18AAP while three ‘drug-like’ and twenty one ‘probe-like’ compounds were identified targeting the PfA-M1, PfA-M17. A secondary assay performed over the 24 hit compounds identified seven compounds that inhibit the activity of PfA-M1, PfA-M17 by >90% while two exhibited >95% inhibition. MMV666023 (151) emerged as potent inhibitor of PfA-M1 with 95% inhibition and reduced the activity of PfA-M17 by 70%. Opposite specificity was observed with MMV020750 (152) which was able to reduce PfA-M17 activity by 95% while reducing PfA-M1 activity to only 75% of total enzyme activity. SAR analysis around 152 investigated the role of phenyl substituent which showed similar inhibition potential of ester and hydroxamic acid as that of parent compound with inhibitory activity in micromolar range against PfA-M1 while >10-fold reduction in activity was observed against PfA-M17. The acid, 153 showed >10-fold increase in activity against PfA-M1. Molecular docking studies further confirmed that compound 151 and 152 are competitive inhibitors where compound 151 act by zinc coordination.93
2.1.4. Dipeptidyl aminopeptidase I (DPAP1). Three classes of endopeptidases like aspartic proteases, cysteine proteases and metalloproteases degrade hemoglobin to oligopeptides in the food vacuole. Although these classes of enzymes are potential targets to induce death of the parasites but the release of small peptides or amino acids from globin oligopeptides requires the services of exopeptidases such as dipeptidyl aminopeptidase 1 (DPAP1) which is located in the food vacuole.94 The dipeptides generated from oligopeptides by DPAP1 forms the substrate for vacuolar aminopeptidase which is converted into single amino acids. Parasite genome encodes two more DPAP enzymes in which DPAP2 is mainly expressed in gametocytes whose function is yet to be explored while DPAP3 is expressed during the erythrocytic cycle of P. falciparum in the active form where it played an essential role in parasite egress from RBCs. DPAPs are considered as potential targets as the closest human ortholog, human cathepsin C is not essential in mammals, although it is involved in activating a family of serine proteases which are involved in inflammation and immune response.95 Since both DPAP1 and DPAP3 play an essential role in parasite life cycle, an inhibitor targeting these proteases will likely to disrupt multiple biological processes.
Validation of DPAP1 as a potentially valuable antimalarial drug target was further carried out. Activity based probes were utilized to reveal that small molecule inhibitors like Ala-4(I)Phe-DMK, 154 caused the formation of an immature trophozoite by specific inhibition of DPAP1 which leads to parasite death (Fig. 21). Although this compound showed potency in vitro but the overall lack of stability prohibited its use for in vivo studies. Therefore, computational methods were explored to design potent non-peptidic inhibitors of DPAP1 that could be evaluated in mouse model of malaria. One of the compound, ML4118S (155) was found to be most potent than its R isomer as the cyclopentane moiety was deeply buried in the S2 pocket while in the R isomer, this group is partially exposed to the solvent. It was effective in killing P. falciparum at single-digit nanomolar conc., stable in mouse serum but toxic in vivo at 20 mg kg−1 and caused a decrease in parasite load in mouse model of malaria. Considering toxicity issues pertaining to this, related compound KB16 (156) which was designed to target Trypanosoma cruzi and known to have low toxicity in mice, showed significant decrease of parasitemia at a dose level of 20 mg kg−1 per day upto day 14. This compound targets berghepains (BP1) with IC50 value of 0.5 μM suggesting that the decrease in parasitemia was due to a combined effect of BP1 and DPAP1 inhibition. Although the compounds reported in this study are associated with lack of potency, poor pharmacokinetic properties and toxicity issues, these results evidenced DPAP1 as viable antimalarial drug target.95
Biochemical properties of DPAP1 were compared with that of human cathepsin C. A method was developed for the production of purified recombinant DPAP1 possessing features similar to those of native enzyme. DPAP1, like cathepsin C is activated by chloride ion and showed its high efficiency in catalysing amide bond hydrolysis at acidic pH values. DPAP1 exhibits monomeric quaternary structure which was different from the homotetrameric structure of cathepsin C. Positional scanning synthetic combinatorial library was profiled to determine S1 and S2 subsite preferences of DPAP1 and cathepsin C by utilizing fluorogenic dipeptidyl-7-amido-4-carbamoylcoumarin (dipeptide-ACC) PSL. The S1 preferences of DPAP1 were found to be similar to other C1-family cysteine peptidases while S2 subsite of both DPAP1 and cathepsin C favoured aliphatic hydrophobic residues, proline and some polar residues, thus exhibiting distinct specificity. Several fluorogenic dipeptide substrates were efficiently catalysed by DPAP1 except a substrate containing P2-phenylalanine residue which was found to be competitive inhibitor. Two reversible cathepsin C inhibitors containing nitrile pharmacophores, 157 and semicarbazide, 158 inhibited both recombinant and native DPAP1 (Fig. 21). Both enzymes were inhibited with sub-nanomolar Ki values for semicarbazide and single-digit nanomolar Ki values for peptide nitrile thus validating recombinant DPAP1 to be used for drug discovery campaign and characterization.94
3. Nucleus
Major pathways of nucleic acid metabolism of Plasmodium are well known targets against antimalarials. The genomic DNA or proteins regulating critical pathways for parasite growth and survival are attractive targets for designing antimalarial inhibitors. Some important enzymes and their inhibitors reported are discussed below.The effect of specific cap group substitution patterns and spacer-group chain lengths were accessed in enhancing the antimalarial and antileishmanial activity of aryltriazolylhydroxamates-based HDAC inhibitors (Fig. 22). The anti-parasitic effect of these compounds well correlated with their anti-HDAC activities. Many of the compounds were several folds cytotoxic selectively to Plasmodium parasite in comparison to standard HDAC inhibitors-SAHA and TSA. These results suggested an optimum length of methylene spacer group being 5 or 6 leading to high activity against protozoa.98
Several PfHDAC inhibitors which were designed to bind the zinc and exterior surface around the active site of the enzyme were reported. 22 compounds possessed cinnamic acid derivatives or NSAID components as HDAC-binding groups while remaining were synthesised by substituting different amines in order to explore active-site space occupation by the 8-aminoquinoline group. Most of the compounds displayed potent antiplasmodial activity with IC50 < 100 nM. All the selected compounds caused hyperacetylation of Pfhistones with >10-fold, more cytotoxicity towards P. falciparum than a normal human cell type. Based on antimalarial and hyperacetylation data, compound 169 may be selected to serve as lead molecule.96
A piggy-back approach was utilised for anticancer histone deacetylase inhibitor SB939 (170) to be developed as potent antimalarial agent. In the biological evaluation against asexual-stage of Plasmodium, it exhibited IC50 values of 0.08 and 0.15 μM against chloroquine-sensitive (3D7) and chloroquine-resistant (Dd2) strains, respectively, causing hyperacetylation of both histone and non-histone proteins of parasite. It exhibited additive effect in combination with the aspartic protease inhibitor lopinavir, 171 while antagonistic effect was observed with primaquine and piperaquine. It also displayed potency against in vitro growth of exoerythrocytic-stage Plasmodium in liver cells with IC50 of 155 nM thus providing additional benefit in targeting multiple life cycle stages of parasite. Evaluation of in vivo efficacy of this compound in an experimental cerebral malaria model revealed significant inhibition of P. berghai ANKA parasite growth with prevention of cerebral malaria-like symptoms. Thus, this study has provided a platform for the development of next-generation anticancer HDAC inhibitors as potential antimalarial drug leads.99
Lack of crystal structures of recombinant PfHDAC made Hansen et al. to establish SAR based on known HDAC inhibitors. The SAR study revealed the importance of cap group, a connecting-unit linker region, as well as a zinc binding group (ZBG). Based on this data, they synthesized 16 HDAC inhibitors bearing alkoxyurea connecting-unit linker region, hydroxamic acid as ZBG and evaluated them for antiplasmodial potential against Pf3D7 strain. Based on the screening of compounds against sexual-stage P. falciparum parasites, hyperacylation tendency of histone H4 for the selected compounds, the compound 172 (Pf3D7 IC50 = 0.16 μm) emerged as most potent asexual stage inhibitor with >30-fold more cytotoxicity in comparison to normal mammalian cells. Thus, 172 can serve as lead for the future development of HDAC inhibitors with improved antiplasmodial activity and enhanced selectivity.97
Having identified the importance of hydroxamic acid as zinc binding group for potent inhibition of PfHDAC, they used human HDAC4 and 5 inhibitor 173, as a starting point for constructing mini-library of HDAC inhibitors containing alkoxyamide connecting-unit linker region. In vitro evaluation of these derivatives against asexual stage P. falciparum revealed IC50 in the range of 0.09–1.12 μM. When examined for HDAC inhibition as well as nuclear lysate % inhibition, compound 174 showed the highest potency with 93.3% and 82.6% inhibition, respectively. Five out of 21 compounds displayed potent inhibition of exo-erythrocytic stage of P. berghai mouse model with IC50 values in the range of 0.16–0.66 μM. Analysis of gametocytocidal data identified 173, 174 and 175 as potent inhibitor with IC50 range 0.25–0.43 μM (Fig. 22).100
Two classes of HDAC inhibitors containing different zinc binding groups (hydroxamic acid vs. thiol) were evaluated for the antiparasitic effect. Hydroxamic acid based HDAC inhibitors emerged as most potent antimalarial compounds with activity ranging from low double-digit to single-digit nanomolar range. Among three derivatives selected on the basis of in vitro evaluation, compound 176 exhibited 88% inhibition of parasitemia in P. berghai mouse model, presenting a new lead for further evaluation.101
Caridha et al. evaluated the potential of substituted thiophene and benzene sulphonamides against malarial and mammalian cyclin dependent protein kinases. Many of the compounds exhibited inhibition of Pfmrk at low to sub-micromolar concentrations. Selected compounds demonstrated specificity for human CDK7 over CDK1, CDK2 and CDK6 with IC50 values in the sub-micromolar range, might be due to the high sequence homology of PfCDK with human CDK7. One of the sub-class of compounds, bromohydrosulfonylacetamides (177) was found to be selective for PfCDK with IC50 value in the submicromolar range (Fig. 23). Compounds tested in the cross-reactivity studies against human CDK7 were also evaluated for antimalarial activity and cytotoxicity studies against mammalian cell lines. These compounds displayed moderate growth inhibition of drug resistant P. falciparum W2 strain as well as low toxicity profiles against mammalian cell lines. Further exploration of bromohydrosulfonylacetamide scaffold with variation in the thiophene and benzene ring substitutions may yield more potent PfCDK inhibitors.104
4. Mitochondria electron transport chain
Mitochondria play a crucial role in the survival of P. falciparum. Mitochondria can be a selective target for the development of antimalarial drugs as several differences exist among mitochondria of parasite and human at molecular as well as functional level. For example, mitochondrial electron transport chain is not essential for ATP production in parasite as their energy demands are met mainly by glycolysis in contrast to human enzyme where oxidative phosphorylation in mitochondria is an essential process for ATP production.105 Mitochondrial electron transport chain is required by parasite for maintaining an intracellular pyrimidine pool for the synthesis of nucleic acids, glycoproteins and phospholipids.106 The mitochondrial electron transport chain consists of five dehydrogenases: NADH: ubiquinone oxidoreductase (PfNDH2), glycerol-3-phosphate dehydrogenase, succinate: ubiquinone oxidoreductase (Complex II or SDH), the malate:quinone oxidoreductase (MQO) and dihydroorotate dehydrogenase (DHODH). PfNDH2 and MQO are absent in human mitochondria while human mitochondrial DHODH exhibit variations at molecular level. These dehydrogenases play a critical role in providing electrons to the downstream complexes: cytochrome bc1complex and cytochrome c oxidase (Complex IV) with ubiquinone (coenzyme Q) and cytochrome c functioning as electron carriers between the complexes. Although the ATP synthase (Complex V) is not involved in the generation of ATP in contrast to its mammalian counterpart, but is involved in the leaking of protons for electron transport chain.107 These apparent differences between parasite and human mitochondria can be exploited for the development of novel chemical scaffolds with acceptable efficacy, pharmacokinetic, toxicity profiles and bioavailability.Twenty-six novel napthoquinone aliphatic esters (rhinacanthin analogues) were synthesised and evaluated them for their antiparasitic effect. Twenty-four esters exhibited substantial antimalarial effect with IC50 values in the range of 0.03–16.63 μM. Length of the chain played an important role in determining the activity as compounds bearing longer chain displayed strong antimalarial activity. Among these, compounds 178 (n = 7), 179 (n = 13) and 180 (n = 15) showed the best activity with IC50 values of 0.133, 0.032 and 0.030 μM, respectively (Fig. 24). Compounds 178 and 179 showed no toxicity against normal Vero cells with high potential therapeutic index of >1990 and 1826, respectively. They also inhibited P. falciparum 3D7 cyt bc1 activity by binding to the Qo site with IC50 values of 5.4 and 8.0 nM respectively as compared to stigmatellin, 181 with IC50 value of 9.1 nM. The results of antiparasitic assay and cyt. bc1 inhibition revealed the importance of 2′-substituents of propyl chain, the length of aliphatic chain, α-methyl substituent of side chain and ester moiety.112
Historical antimalarial compound endochin, 182 was utilised as a structural lead for optimization and adopted a novel chemical route to synthesize endochin-like quinolones (ELQ) i.e. 4(1H)-quinolone-3-diarylethers. Evaluation of these derivatives against multidrug resistant strains of P. falciparum and in vivo efficacy identified compound 183 (ELQ-300) as the most potent inhibitor of P. falciparum strains D6, Dd2, Tm90-C2B. Moreover, compound 183 exhibited excellent in vivo potency against P. yoelii in mice with ED50 and ED90 values of 0.02 and 0.06 mg kg−1 per day, respectively, and a non-recrudescence cure dose of 0.3 mg kg−1 per day. Noteworthy feature of 183 was its 30-fold more potency than atovaquone against murine malaria. It was found to be ≥ 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold more selective for parasite bc1 complex over human enzyme with EC50 value of 0.56 nM thus exhibiting superiority over atovaquone (IC50 = 2.0 nM). Preclinical animal studies on 183 demonstrated nonlinear pharmacokinetics i.e. good to excellent oral bioavailability at low doses needed for therapeutic effect but fell off rapidly at higher doses. Further studies need to be carried out to identify clinical formulation in order to improve exposure at higher doses. Compound 183 displayed potential that might be carried forward for clinical studies.113
000-fold more selective for parasite bc1 complex over human enzyme with EC50 value of 0.56 nM thus exhibiting superiority over atovaquone (IC50 = 2.0 nM). Preclinical animal studies on 183 demonstrated nonlinear pharmacokinetics i.e. good to excellent oral bioavailability at low doses needed for therapeutic effect but fell off rapidly at higher doses. Further studies need to be carried out to identify clinical formulation in order to improve exposure at higher doses. Compound 183 displayed potential that might be carried forward for clinical studies.113
A series of 3-alkyl-2-hydroxy-1,4-naphthoquinones was evaluated for its antiparasitic effect. Four compounds exhibited IC50 in the mid micromolar range with one of them, 184 displayed IC50 in the nanomolar range (IC50 = 443 nM). This most active inhibitor reportedly disrupted mitochondrial membrane potential with IC50ΔΨmt = 16 μM and displayed low cytotoxicity against human cells which advocate its potential as an antimalarial agent.114
A new class comprising tetracyclic benzothiazepines (BTZs) was discovered as highly active and selective antimalarials with potency in nanomolar range. SAR trend among these derivatives (185–190) exhibited the importance of small hydrophobic substituents at R2 site and non-polar aliphatic groups at R1 site in enhancing antimalarial activity (Fig. 24). Synergistic studies of 185 with proguanil produced results comparable to that of atovaquone. Validation of cytochrome bc1 as the target for this class was established on the basis of the combined approach employing genetically modified parasites, biochemical profiling, and resistance selection. As the resistance to atovaquone is reducing its efficacy, this class, being able to retain its potential against atovaquone resistant parasites, can be used as an alternative to atovaquone in combination therapy.115
A series of hydroxy-naphthoquinones possessing a methyl group on the benzene ring was synthesised and tested for interaction with the nuclear encoded rieske iron-sulphur protein (Fig. 24). Evaluation of these compounds against yeast and bovine cytochrome bc1 complex as a model of parasite and human enzyme, respectively identified 191 as a potential inhibitor. The metabolic instability associated with cytochrome bc1 inhibitor, S-10576 (192) has been reported to overcome by introducing the trifluoromethyl function at the terminal end. The specificity of inhibitors for yeast bc1 complex over bovine bc1 revealed less selectivity than 192 but higher as compared to atovaquone.116
Cowley et al. prepared a series of quinolones substituted at 6- and 7-position utilizing Gould–Jacobs approach. The 7-substituted analogues expressed activity at as lower range as 0.46 nM against P. falciparum. SAR data of this series of compounds suggested the importance of ester functionality at 3 position and the presence of benzyl linker. The exceptional potency of compound 193 was further explored as cytochrome bc1 inhibitor. Cytochrome c reductase assay revealed that potency of this compound (EC50 = 1.3 nM) lies in the same region as that of atovaquone (3.0 nM). Although the compounds displayed high potency, the problem associated with their solubility needs to be addressed.108
A novel class of conjugated quinoline-indoles which exhibited potent activity against P. falciparum was reported. Compounds bearing quaternary nitrogen on the quinoline (194 and 195) showed enhanced activity against the chloroquine-resistant K1 strain while compounds possessing non-quaternerized nitrogen (196) are somewhat less active against K1 strain but these showed improved potency against chloroquine sensitive 3D7 strain in comparison to their analogues (Fig. 24). The antimalarial data clearly focused on the importance of conjugated indole ring in maintaining its antimalarial potential. It was suggested that quinoliniums showed less potency when parasites were exposed to target compounds for a short period of time. The mechanism of action of these compounds was reportedly due to the disruption of mitochondrial potential as evident from the structural relationship between these compounds and pyrivinum pamoate, which is supposed to act via dissipating mitochondrial function. These compounds disrupted mitochondrial potential at the conc. similar or even less than that required for parasite killing.117
Nam et al. identified decoquinate, 197 through a high-throughput screening of an annotated compound library comprising >28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds possessing drug-like characteristics. In vitro evaluation of decoquinate against blood stage P. falciparum exhibited activity in the single-digit nanomolar range with IC50 value of 4.3 nM. A chemical genomic analysis of decoquinate-resistant line revealed that resistance was conferred by mutations in cytochrome b. In addition to that, decoquinate was also found active in vitro against liver stage P. yoelii with IC50 value of 177 pM and when adminstered to mice at dose level of 50 mg kg−1 provided partial prophylaxis protection. The insensitivity of transgenic parasites expressing yeast DHODH to decoquinate further evidenced that actual target of this drug is mitochondrial electron transport chain. Molecular modelling studies revealed distinct mode of binding within the ubiquinol-binding site of cytochrome b for both decoquinate and atovaquone which formed the basis for limited cross-resistance of decoquinate to a panel of atovaquone-resistant parasites.118
000 compounds possessing drug-like characteristics. In vitro evaluation of decoquinate against blood stage P. falciparum exhibited activity in the single-digit nanomolar range with IC50 value of 4.3 nM. A chemical genomic analysis of decoquinate-resistant line revealed that resistance was conferred by mutations in cytochrome b. In addition to that, decoquinate was also found active in vitro against liver stage P. yoelii with IC50 value of 177 pM and when adminstered to mice at dose level of 50 mg kg−1 provided partial prophylaxis protection. The insensitivity of transgenic parasites expressing yeast DHODH to decoquinate further evidenced that actual target of this drug is mitochondrial electron transport chain. Molecular modelling studies revealed distinct mode of binding within the ubiquinol-binding site of cytochrome b for both decoquinate and atovaquone which formed the basis for limited cross-resistance of decoquinate to a panel of atovaquone-resistant parasites.118
da Cruz et al. also identified 197 via the screening of a library containing 1037 existing drugs with the ability of inhibiting Plasmodium hepatic development. Compound 197 displayed highest potency against liver stages, both in vitro and in vivo. Besides the ability to inhibit Plasmodium liver stages, it was also reported to be effective in killing the parasite's replicative blood stages and is potent against developing gametocytes inhibiting its transmission. The mode of action was demonstrated by exploring Plasmodium transfected with yeast DHODH which revealed that it causes selective and specific inhibition of parasite's mitochondrial bc1 complex with an IC50 of 0.002 μM and selectivity index of >5000 in comparison to human bc1 complex and showed little cross-resistance with antimalarial drug atovaquone. Single dose of decoquinate (197) is highly effective in preventing the appearance of the disease when administered orally thus presenting a highly potent and highly effective antimalarial drug targeting multiple stages of the parasite.119
A new family of plasmodial cytochrome bc1 inhibitors was reported based on 4(1H)-pyridone scaffold which displayed potent antimalarial activity against P. falciparum in vitro and in vivo. The mechanism of action of this new family was found to be selective inhibition of electron-transport chain of P. falciparum targeting mainly cytochrome bc1 complex. Inspite of similar mechanism of action to that of atovaquone, 4(1H)-pyridones did not exhibit cross-resistance with the former indicating that two exert different binding interactions in the cytochrome bc1 complex. The lead optimization studies focused on improving potency and physicochemical properties, discovered a highly active antimalarial candidate, GSK932121 (198) which has been carried forward for human trials. Nonetheless, soluble phosphate prodrug, 199 of the candidate produced toxicity in the rat which led to the termination of 198 in human trials demonstrating a possibly narrow therapeutic index (Fig. 24).106
The effect of substituents within the N-arylaminomethylene malonate inhibitors was studied in terms of PfDHODH affinity and selectivity over that of human enzyme. Further the effect of flexibility associated with the diethyl portion of the molecules was also studied by conversion of free alkyl groups of ester groups into a rigid cyclic framework. Analysis of enzyme inhibition results along with molecular modelling predictions suggested the importance of diester head groups where both the ester groups bind in a conformation in which the alkyl groups of the ester moiety have opposite orientations.123
A series of N-alkyl-5-(1H-benzimidazol-1-yl)thiophene-2 carboxamides was identified which exhibited inhibition of PfDHODH in low nanomolar range and selectivity over hDHODH (Fig. 26). These compounds also displayed antiparasitic effect against 3D7 and Dd2 strains as well as good tolerability and oral exposure in the mouse with ED50 values of 13–21 mg kg−1 per day in the 4 day murine P. berghei. Although compound Genz-667348 (204) was found active, it exhibited considerable inhibition of CYP2D6 as well as the cardiac hERG channel that makes it less desirable to be developed as antimalarial candidate. Analogs Genz-668857 (205) and -669178 (206), based on equipotency and favourable cytochrome P450 and hERG inhibition can be further tested to determine their candidature for preclinical development.124
Gujjar et al. carried out the optimization studies on the lead inhibitor possessing triazolopyrimidine core that has been identified previously.125 Lead optimization study result in the identification of inhibitors, 207, 208 bearing 4-SF5 and 3,5-di-F-4-CF3 substituted aniline moieties coupled to triazolopyrimidine ring, respectively. These inhibitors were superior to previously reported inhibitors125 in terms of plasma exposure and efficacy in the P. berghei mouse model.126
PfDHODH inhibitors were identified through structure-guided lead optimization around previously identified triazolopyrimidine scaffold. These derivatives were modified at the C2 position of the basic core. Two compounds, 209 and 210 were found to be potent against both sensitive and drug resistant strains of the parasite. The compound 210 showed similar potency to chloroquine in the humanized SCID mouse P. falciparum model. The compounds demonstrated excellent oral bioavailability, long half-life and low clearance. Based on enhanced in vivo efficacy as well as more linear pharmacokinetics, 210 has been chosen to be developed as clinical candidate. High resolution crystal structure of DHODH bound to 210 revealed that inhibitor 210 forms two hydrogen bonds with Arg265 and His185 in the binding pocket located between FMN and the N-terminal α-helix (Fig. 25).121
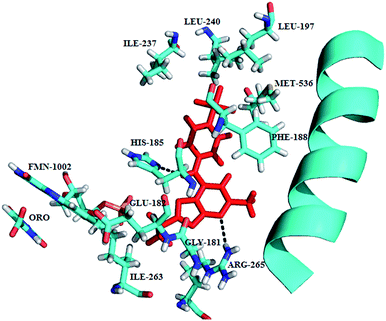 | ||
| Fig. 25 X-ray structure of inhibitor binding site of PfDHODH bound to 210. Protein, falvin mononucleotide (FMN) (carbons in cyan); 210 is shown in red and hydrogen bonds are shown in black broken lines. The figure was made by using PyMol (PDB ID: 5BOO). | ||
Skerlj et al. synthesized and optimised a series of 5-(2-methylbenzimidazol-1-yl)-N-alkylthiophene-2-carboxamides, that are reported as promising PfDHODH inhibitors for the development of potent antimalarial agents (Fig. 26). The selectivity over human enzyme, efficacy in the three mouse models, acceptable safety pharmacology risk assessment and safety toxicology profile in rodents, lack of potential drug–drug interactions, acceptable ADME/pharmacokinetic profile, and projected human dose formed the basis for the selection of compound 211 to be developed as potential candidate.127
A series of N-substituted salicylamides was synthesised and evaluated for PfDHODH inhibition potential. The enzymatic assay revealed that selectivity as well as potency can be increased by introducing a 2,2-diphenylethyl substitution on the salicylamidic nitrogen (Fig. 26). The inhibition assay identified compound 212 as the most potent PfDHODH inhibitor with IC50 of 7.0 μM. The active compounds were further tested for their potential in whole cell assay and compound 213 was found to inhibit parasite growth with EC50 value of 23 μM.128
Marwaha et al. identified imidazo[1,2-a]pyrimidines pharmacophore as potent PfDHODH inhibitors based on bioisosteric transformations and permutations in the triazolopyrimidine scaffold reported previously. Depending upon the nature of amino aniline substitution, these compounds found to exhibit more potency than the triazolopyrimidines. The most potent candidate, 214 in this series displayed 4-fold better affinity (IC50 = 0.077 μM) compared to the equivalent triazolopyrimidine analog. The in vivo potential of the active candidate demonstrated 56% suppression of the parasites which was lower than the triazolopyrimidine analogs in the mouse model. This study showed the importance of N1, N4 and N5 functional sites which should be taken care of for the development of more effective antimalarial agents.129
A novel series of dihydrothiophenone derivatives as PfDHODH inhibitors were identified through virtual screening. Further structural optimization and SAR analysis of dihydrothiophenone derivatives led to the discovery of various potent and specific PfDHODH inhibitors. The most favorable compound 215, comprising dihydrofuranone ring, exhibited selective inhibition of PfDHODH (IC50 = 6.0 nM) with >14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold selectivity over hDHODH. The in vitro parasite growth inhibition provided encouraging results with IC50 of 15 and 18 nM against 3D7 and Dd2 cells, respectively. Furthermore, in vivo pharmacokinetics studies revealed 40% oral bioavailability for compound 215. Further improvement of pharmacokinetic properties is required for this series to work on.130
000-fold selectivity over hDHODH. The in vitro parasite growth inhibition provided encouraging results with IC50 of 15 and 18 nM against 3D7 and Dd2 cells, respectively. Furthermore, in vivo pharmacokinetics studies revealed 40% oral bioavailability for compound 215. Further improvement of pharmacokinetic properties is required for this series to work on.130
A range of ligand-based chemoinformatic methods were used to identify selective inhibitor of P. falciparum type II NADH:quinone oxidoreductase (PfNDH2). Extensive structural investigation led to the selection of series of 2-bisaryl 3-methyl quinolones for further antimalarial evaluation (Fig. 27). Several compounds of this series exhibited selective inhibition of PfNDH2 enzyme. The most potent compound 216 (CK-2-68) with IC50 of 36 nM against 3D7 strain of P. falciparum, was reported to selectively inhibit PfNDH2 enzyme as compared to other respiratory enzyme with IC50 of 16 nM. The most important feature of this lead compound was its low cytotoxicity as well as its high metabolic stability in the presence of human liver microsomes. The prodrug of lead compound 216 was also found to be effective at clearing parasitic infection after oral administration at 20 mg kg−1 in murine model of malaria. Other quinolones in this series (217, 218 and 219) displayed dual targeting of key mitochondrial enzyme PfNDH2 and P. falciparum cytochrome bc1 in the low nanomolar range presenting additional advantage over single-targeted inhibitors.133 Lead optimization studies on compound 216 (CK-2-68) was carried out in order to reduce clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and improve solubility by incorporating a variety of heterocycles within the side chain of quinolone core. This strategy led to the identification of a lead compound SL-2-25 (220) which exhibited both the enzyme inhibition (PfNDH2) and whole cell antiparasitic activity (3D7 strain of P. falciparum) in the nanomolar range with IC50 15 nM and 54 nM, respectively. It also displayed remarkable oral activity of ED50/ED90 of 1.87/4.72 mg kg−1 against P. berghei (NS strain) in murine model of malaria when administered as a phosphate salt. Some of the analogues in this series (220, 221 and 222) provided additional benefit being the dual inhibitor of PfNDH2 and cytochrome bc1 (Fig. 27).134
P and improve solubility by incorporating a variety of heterocycles within the side chain of quinolone core. This strategy led to the identification of a lead compound SL-2-25 (220) which exhibited both the enzyme inhibition (PfNDH2) and whole cell antiparasitic activity (3D7 strain of P. falciparum) in the nanomolar range with IC50 15 nM and 54 nM, respectively. It also displayed remarkable oral activity of ED50/ED90 of 1.87/4.72 mg kg−1 against P. berghei (NS strain) in murine model of malaria when administered as a phosphate salt. Some of the analogues in this series (220, 221 and 222) provided additional benefit being the dual inhibitor of PfNDH2 and cytochrome bc1 (Fig. 27).134
A potential inhibitor of P. falciparum cell proliferation, 1-hydroxy-2-dodecyl-4(1H) quinolone (HDQ), 223 that inhibited parasite bc1 complex from both control and atovaquone-resistant strains TM90C2B with IC50 values in the submicromolar range (19 nM and 64 nM, respectively) was reported. The inhibition data clearly indicated that the drugs, atovaquone and HDQ targets different sites within the complex. In addition, HDQ also inhibited recombinant PfNDH2 with IC50 value of 77 nM. To further determine the binding site of HDQ, yeast model was used to introduce point mutations into the Qi and Qo site. Point mutations introduced in the Qi site significantly reduced HDQ inhibition while it did not affect HDQ potency when inhibitor resistance mutations were introduced. This extensive study for the exploration of the mode of action of HDQ indicated that Qi site of the bc1 complex can be a target for antimalarials.110
5. Apicoplast
P. falciparum contains a characteristic non-photosynthetic plastid-like organelle called apicoplast. This organelle has homology with the chloroplasts of plants and algae and is surrounded by four membranes. P. falciparum apicoplast, like other plastids possess its own genome that encodes for transcription and translation related genes135 and carries out crucial processes bearing similarities to those of plant plastids and cyanobacteria such as fatty acid synthesis type II (FAS-II) pathway, a non-mevalonate (DOXP) pathway of isoprenoid biosynthesis, iron-sulphur clusters and heme synthesis. The metabolites and cofactors produced by these pathways are required for a variety of cellular processes and crucial for parasite survival thus, apicoplast is considered indispensable for parasite and can be exploited for antimalarial drug discovery campaign.136Gupta et al. generated a common feature pharamcophore consisting of two hydrogen-bond acceptor and one aromatic hydrophobic feature using seven active flavonoids. In vitro evaluation of these compounds against FabI inhibition and growth inhibition of chloroquine sensitive (NF-54) P. falciparum strain suggested that (−)-catechin gallate, 224 was the most active compound with IC50 values of 0.3 μM and 3.2 μM, respectively (Fig. 29). Docking analysis also validates the pharmacophore model which is quite useful for the design of potent FAS-II inhibitors.139
Thirteen bromopyrrole alkaloids from marine sponges belonging to Axinella and Agelas genera were isolated and evaluated for their potential against four parasitic protozoa i.e. T. brucei rhodesiense, T. cruzi, Leishmania donovani and P. falciparum K1 strain. The results reported longamide B (225) (IC50 = 1.53 μg ml−1) and dibromopalau'amine (226) (IC50 = 0.46 μg ml−1) to be promising trypanocidal agents (Fig. 29). Both these alkaloids exhibited exciting antileishmanial activities with IC50 values of 3.85 and 1.09 μg ml−1, respectively. Dispacamide B (227) (IC50 = 1.34 μg ml−1) and spongiacidin B (228) (IC50 = 1.09 μg ml−1) exhibited selective antimalarial activity and SI values of >67.2 and 32.7, respectively. The inhibition assay against enzymes involved in fatty acid biosynthesis pathway identified bromopyrrolohomoarginin (229) as a potent inhibitor of PfFabZ with IC50 = 0.28 μg ml−1. Since its potential is identical to (−)-epigallocatechin gallate, 230 (IC50 = 0.32 μg ml−1), it can be further explored as an antimalarial agent.140
2-Hexadecynoic acid, 231 was reported as the novel inhibitor of plasmodial FAS-II enzymes having capability to arrest erythrocytic and liver stage Plasmodium infections. The in vitro evaluation of 2-, 5-, 6- and 9-hexadecynoic acids (HDAs) against erythrocytic stages of P. falciparum and liver stages of P. yoelii infections indicated highest antiplasmodial activity of 5-HDA, 232 (IC50 = 6.6 μg ml−1) against blood stages of P. falciparum. Compound 231 was reported to be responsible for arresting the growth of liver stage P. yoelii infection indicated by both flow cytometric assay (IC50 231 = 15.3 μg ml−1, control drug atovaquone = 2.5 ng ml−1) and immunofluorescence analysis (IC50 231 = 4.88 μg ml−1, control drug atovaquone = 0.37 ng ml−1). The inhibitory activity of HDAs was also evaluated against multiple P. falciparum FAS-II (PfFAS-II) elongation enzymes and reported that 231 displayed best inhibitory activity against the PfFAS-II enzymes PfFabI, PfFabZ and PfFabG with IC50 values ranging from 0.38–3.50 μg ml−1. Kinetic studies and molecular modelling studies predicted the inhibitory mechanism of 2-HDA. Kinetic studies revealed the inhibition of PfFabI was non-competitive with respect to crotonyl-CoA and the cofactor NADH which implies the binding of 2-HDA to the enzyme at a different site that of substrate and the cofactor site. Thus, 231 could be developed as potent FAS-II inhibitor focusing mainly on the presence of a triple bond next to the carbonyl group which increases the conformational flexibility and dipole moment of 2-HDA, enabling it to interact with PfFAS-II target enzymes more efficiently.141
Coumarin-based triclosan analogues were developed as novel inhibitors of PfFabI. Among all the tested compounds, 233 and 234 exhibited the highest inhibitory activity with IC50 values of 0.25 and 0.27 μM, respectively. Compound 235 emerged as potent dual PfFabI/PfFabZ inhibitor, targeting two enzymes in the same pathway with IC50 value of 0.45/10 μM thus focusing on the importance of 2-hydroxy-4-chlorophenyl-based diaryl ether framework, as a capping fragment, for the inhibition of both Pf FabI and parasite growth. X-ray data revealed the interaction of coumarin-based triclosan analogues to the NADH binding pocket interacting with Ala219 backbone and Asn218 side chain (Fig. 28).142
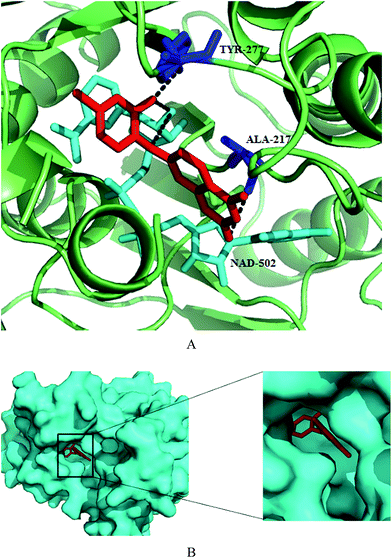 | ||
| Fig. 28 Structural details of substrate/enzyme binding site. (A) Protein structure is shown in green colour. Catalytic site residues and cofactor NADH are depicted in blue and cyan colour, respectively while the inhibitor is shown in red colour. (B) Surface representation of protein (cyan colour) showing inhibitor in the active site. Figure was drawn in PyMol using PDB ID 4IGE. | ||
A new methylene-bridged bisflavonoid, methylenebissantin (237) was isolated along with nine known compounds, including flavonoids, diterpenoids, and phenol derivatives from the aerial parts of Dodonaea viscosa Jacq. These isolates were evaluated for the PfENR inhibition in which 237 exhibited moderate inhibition of the enzyme with IC50 value of 91.13 μM.143
Lauinger et al. evaluated four lichen metabolites evernic acid, 238, vulpic acid, 239, psoromic acid, 240 and (+)-usnic acid, 241 against FAS-II enzymes (Fig. 29) in which evernic acid emerged as potent PfFabZ inhibitor with IC50 value of 10.7 μM and compound 241 was found to be most potent against LS activity and also displayed stage specificity (LS IC50 value 2.3 μM and BS IC50 value 47.3 μM). Lichen acid binding to allosteric site affected the enzyme conformation thus, influencing the enzyme activity.137
Marchantin A, 242, a macrocyclic bisbibenzyl ether, was isolated from the liverwort Marchantia polymorpha, and tested its potential against the three recombinant enzymes (PfFabI, PfFabG and PfFabZ) of the PfFAS-II pathway of P. falciparum. The results demonstrated moderate inhibiton against PfFabZ (IC50 = 18.18 μM) while PfFabI, PfFabG enzymes were not affected. The cytotoxicity results against primary L6 rat cells with CC50 value of 6.64 μM suggested low selectivity index (SI) in vitro. Inspite of that, it has been found to have low toxicity in vivo and can be considered for further investigation and development to improve the selectivity and activity against the FAS-II enzyme pathway. Docking study predicted that in comparison to small molecule inhibitors which can enter narrow active site tunnel of the enzyme, marchantin A, (242), being bulky, supposed to block the entrance of the tunnel thus inhibiting the substrate from reaching the active site tunnel.138
Based on the virtual screening of iResearch database by molecular docking, a new class of phenylaminoacetic acid benzylidene hydrazines as inhibitor of PfENR was developed by Samal et al.144 The spectrophotometric assay to screen the compounds in vitro against recombinant PfENR enzyme revealed 243 and 244 as potent PfENR inhibitor with IC50 values 8.0 and 7.0 μM, respectively followed by compound 245 (IC50 = 10 μM) and 246 (IC50 = 12 μM) (Fig. 29). This suggests that the presence of electron withdrawing group at para position is more preferred over electron releasing group that correlates to the greater inhibition potential.
The work on 2-alkynoic fatty acids was extended further for the search of more potent inhibitor of plasmodial FAS-II enzymes and reported 2-octadecynoic acid, 247, as best inhibitor of P. berghei parasites with ten times higher potency (IC50 = 0.34 μg ml−1) than the control drug primaquine. It was also found to be most active against blood stages of P. falciparum with IC50 value of 6.0 μg ml−1 thus acted as dual life stages inhibitor of Plasmodium species. The target determination studies revealed that the same compound effectively inhibited three P. falciparum FAS-II (PfFAS-II) elongation enzymes PfFabI, PfFabZ, and PfFabG with the lowest IC50 values (0.28–0.80 μg ml−1). The better inhibitory potential of compound 247 against PfFAS-II enzymes than 231 laid emphasis on right alkyl chain length (longer) coupled with triple bond at C-2 for the inhibition of both liver stage parasites growth and target enzyme activity.145 Celastrol, 248 a triterpene quinone methide was isolated from the roots of the celastraceae Tripterygium wilfordi. Enzymatic assay revealed that celastrol inhibits PfENR in a non-competitive manner with average IC50 values of 5.9 and 4.7 μM with and without NAD + preincubation, respectively.146
Reverse analogues of 249 displaying high activity and selectivity for PfDXR inhibition were evaluated for recombinant DXR inhibition as well as in vitro antiplasmodial activity against the multidrug-resistant K1 strain of P. falciparum. N-Methyl substituted hydroxamic acid 251 emerged as most potent PfDXR inhibitor with IC50 value of 3.1 nM, even more potent inhibitor than the reference fosmidomycin (249) and FR900098 (250) (Fig. 31). These compounds were found to be non-cytotoxic on human MRC-5 cells. Thus, the high potency of these compounds shed light on the importance of α-phenyl substitution and the reverse orientation of the hydroxamic acid group.151
Reverse fosmidomycin derivatives as inhibitors of DXR (IspC) were synthesized and biologically evaluated by Behrendt et al. Most of the compounds inhibited the target protein (PfDXR) with IC50 values in the low nanomolar range. The most active compounds 252, 253 exhibited the IC50 value of 3.0 nM against DXR enzyme as well as strong potency against P. falciparum replication in erythrocytes in vitro thus emphasizing the importance of free as well as N-methyl substituted hydroxamic acid and the correct selection of aryl substituents in the α-position. In vivo studies on P. berghei mouse model have shown that the compound 253 displayed 78% suppression of infected RBCs but with low % of survival rate. While the compound 252 with 89% suppression of infected RBCs with almost 60% survival rate has shown some potential and can be developed as promising drug candidate by further structural modifications and pharmacological studies.152
Brücher et al. developed α-aryl-substituted β-oxa isosteres of fosmidomycin with a reverse orientation of the hydroxamic acid group. These were evaluated for their inhibitory potential against recombinant PfDXR (IspC) and in vitro antiplasmodial activity against chloroquine-sensitive and resistant strains of P. falciparum. Although oxa compounds showed weaker inhibitory activity as compared to cognate carba compounds, compound 254 displayed high potency against DXR protein of P. falciparum with an IC50 value of 12 nM as well as potent in vitro antiplasmodial activity. In addition, lipophilic ester prodrugs of compound 254 demonstrated improved efficacy in P. falciparum growth assays with better IC50 values (0.013–0.031 μM) than 249. Thus, the combination of methylene group in the β-position and methyl group at the hydroxamic acid motif produces inhibitors with the highest affinity (Fig. 31).153
Novel non-cytotoxic pyridine-containing fosmidomycin derivatives were developed as highly potent inhibitors of PfDXR. They showed Ki values ranging from 1.9 to 13 nM, with the best compound 255 (Ki = 1.9 nM) possessing an acetyl and a pyridin-3-yl group which was 11 times more active than fosmidomycin. A 2.3 Å crystal structure of PfDXR in complex with one of the inhibitors 256 has been reported, showing that conformational changes takes place especially in the flexible loop at the active site of the enzyme upon ligand binding (Fig. 30). Moreover, a hydrogen bond along with favorable hydrophobic interactions between the pyridine group and the PfDXR account for the enhanced activity. Thus, these novel inhibitors emphasized the importance of the presence of an electron-deficient aromatic ring, such as a pyridine, at the α-position of the phosphonate group.149
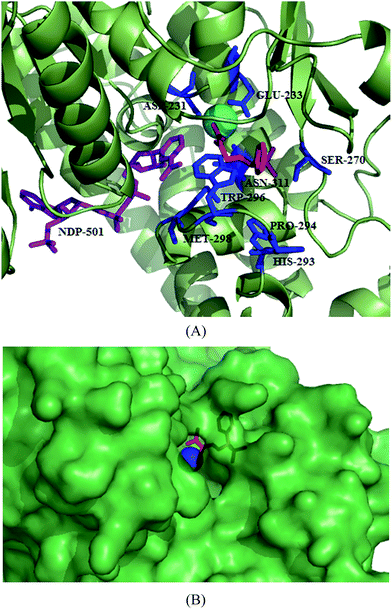 | ||
| Fig. 30 (A) Crystal structure of PfDXR in complex with Mn2+ (cyan sphere), 256 (red in colour) showing close-up view of the active site of PfDXR: 256. Active site residues and the cofactor are shown in blue and purple colour, respectively (B) Surface representation of PfDXR: 256 complex with Mn2+ (blue sphere). Figures were drawn using PyMol (PDB ID: 4GAE). | ||
A series of aryl and heteroarylcarbamoylphosphonic acids, their diethyl esters and disodium salts as analogues of the potent DXR inhibitor fosmidomycin has also been reported. They were evaluated for the inhibitory potential against PfDXR as well as against Pf3D7 strain growth and for human cell toxicity. Out of these, 257 was found to inhibit the activity of both PfDXR, EcDXR enzymes and Pf3D7 strain; and also non-toxic against human cell line Hst578T. Enzyme inhibition assay as well as docking studies indicated optimal linker length of two methylene units.150 Thia isosters of reverse hydroxamic acid analogues of fosmidomycin were synthesised and found that N-methyl substituted hydroxamates are more potent than their respective unsubstituted hydroxamic acids, supporting the previous observation by other groups. In comparison to its oxa and carba compounds, thia isosters were found to be less potent inhibitor of PfDXR. The results of in vitro antiplasmodial activity were not well correlated with that of enzyme inhibition due to the fact that the compounds have to cross several parasitic membranes to reach its site in apicoplast. The crystal structure determined with 258 was similar to the previously reported PfDXR structures. It predicted that loop region over the active site can accommodate more bulky residues like phenyl and naphthyl group due to high mobility. To clarify the long unresolved question pertaining to the stereochemistry of chiral IspC ligands, enantiomers of most active compound 258 were separated by chiral HPLC and tested for enzyme inhibition potential against PfDXR found to possess high degree of enantioselectivity towards enantiomer S-(+)-258 with IC50 value of 9.4 nM (Fig. 31).154
To gain insights into the SAR of reverse fosmidomycin analogues, Konzuch et al. synthesized fosmidomycin analogues with reverse orientation by modifying key regions. The synthesised compounds were evaluated for their enzyme inhibition potential as well as their growth inhibitory effect on asexual stage P. falciparum. Reverse carba- and oxa-analogs were found to be more potent P. falciparum growth inhibitors. The enzyme inhibition results clearly indicated the importance of aryl group at α-position of fosmidomycin in increasing the potency against PfDXR. The most active compound 259 exhibited IC50 in low nanomolar range. Crystallographic studies revealed the enantioselectivity of S-enantiomers of α-aryl substituted carba- and oxa-analogs of fosmidomycin in the active site of PfDXR.155
To investigate the effect of N-acyl substitution on the PfDXR, Cobb et al. carried out enzymatic biosynthesis of FR-900098 (250) analogues and based on the co-crystal structures of PfDXR with fosmidomycin and FR-900098, N-propionyl derivative FR-900098P, 260 was selected for PfDXR inhibition and it was found to be more potent than the parent compounds with Ki value of 0.92 nM. Based on the improved inhibition potential, in vivo biosynthetic platform was developed for its synthesis employing both mutasynthetic and metabolic engineering strategies representing a sustainable and environment compatible methodology.156
To further improve the pharmacodynamics and pharmacokinetics of 249, Brücher et al. synthesized its prodrugs with reverse orientation. The modification of phosphonate moiety of fosmidomycin to its acyloxymethyl and alkoxycarbonyloxymethyl ester groups and esterification of hydroxamate moiety yielded double prodrugs. These prodrugs were evaluated for DXR inhibition potential as well as antiplasmodial in vitro activity against asexual blood stages of P. falciparum. None of the prodrugs (phosphonate and phosphonate–hydroxamate double prodrugs) exhibited considerable enzyme inhibition due to their bulkiness and inability of double prodrugs to chelate essential metal ion. The better part of the whole study was the improved antiplasmodial in vitro activity of the prodrugs in comparison to their parent compounds with IC50 value in the nanomolar range. Among all the studied compounds, the most potent prodrug was found to be 261 with IC50 value against PfDd2 was 4.0 nM and the best results were obtained with n-butyloxycarbonyloxymethyl group. Although the compounds displayed better in vitro efficacy but the selected compounds failed to produce significant in vivo results.157
To evaluate the effect of substituted aromatic groups at β-position of the propyl backbone of N-methyl substituted reverse fosmidomycin analogue on DXR inhibition, Chofor et al. synthesized for the first time β-substituted fosmidomycin analogues (Fig. 31). It was found that the direct substitution of aryl moiety at β-position produced poor inhibitor while the longer length of linker between the carbon backbone and the phenyl ring is responsible for better binding to the target enzyme. These derivatives were also evaluated for their in vitro schizontocidal activity against the P. falciparum K1 strain and the results were well correlated with the PfDXR inhibitiory activity and recommends that four carbon chain (262) is optimal for enhanced PfDXR inhibition.148
A set of diverse compounds were screened from the Pfizer corporate collection against P. falciparum and L. donovani NMTs. These selected hits were further tested for their selectivity over both human isoforms (Hs1 and Hs2) and for broad-spectrum anti-protozoan activity against T. brucei NMT. Analysis of screening results revealed the distinct SAR for Leishmania NMT from all other NMTs tested while a strong overlap was observed between SARs of Plasmodium NMT and both human NMTs posing challenge to achieve selectivity profile. Nonetheless, the group discovered two novel series targeting selectively Plasmodium NMT over other NMT orthologues and two structurally divergent series with selectivity over Leishmania NMT.162
Potent and selective inhibitors of P. falciparum NMT based on compound 266, were designed and synthesized possessing benzofuran scaffold, identified by screening a focused library of NMT inhibitors of C. albicans NMT (CaNMT) and T. brucei NMT (TbNMT) by following “piggy-back” approach. Chemistry-driven optimization approach was carried out on compound 266 to identify two lead compounds 267 and 268 with submicromolar IC50 value of 0.27 and 2.0 μM, respectively (Fig. 32). These potent PfNMT inhibitors have displayed highest antiparasitic activity as well as excellent selectivity over human NMT. These findings highly recommend the development of the optimized PfNMT inhibitors into antimalarial therapeutics. The crystal structure data of PvNMT in complex with compound 269 rationalized the superiority of benzyl ester side chain and piperidine substituent as well as determines the interactions involved with the linker. This suggests that replacing the aromatic R2 group by N-containing heterocycles can further improve the enzyme activity.160
In continuance to the discovery of P. falciparum and P. vivax N-myristoyltransferase inhibitors, Rackham et al. designed and synthesised novel benzo[b]thiophene-containing inhibitors by utilizing lead-hopping approach. Among all the compounds, 270 exhibited higher PfNMT affinity, selectivity against HsNMT, excellent ligand efficiency and markedly improved antiparasitic activity than benzo[b]furan analog. Analysis of SAR in the series yielded compound 271 which displayed potent PvNMT enzyme affinity and 10-fold selectivity over PfNMT but it was found to be selective for HsNMT. This was found to be the most potent antiplasmodial compound among the series with EC50 value of 2.0 μM. This observation was rationalized from the crystal structure of PvNMT bound to 271 which shows the altered substitution pattern of benzothiophene which is deeply buried in the hydrophobic pocket.163
Benzo[b]thiophene scaffold based high affinity inhibitors of P. falciparum and P. vivax NMT based on Ligand Efficiency Dependent Lipophilicity (LELP) were further developed. This approach guided the discovery of compound 272 with 100-fold increase in enzyme affinity and 100-fold reduction in liphophilicity due to the addition of only two heavy atoms. This compound exhibited potency against resistant strains, blood and liver stage forms as well as selectivity over human liver host cells.159
Olaleye et al. developed peptidomimetic inhibitors of Plasmodium and Leishmania NMT based on C. albicans (CaNMT) peptidomimetics, as P. vivax and L. donovani NMTs are reported to have 44% and 43% sequence identity with CaNMT, respectively. Peptidomimetic scaffolds were designed by employing modifications at the C- and N-termini thereby exploiting both the ends. Among all the tested compounds, amine 273 displayed significantly improved potency against the NMTs of P. vivax (PvNMT), L. donovani (LdNMT) and H. sapiens (HsNMT1). While the compound 274, obtained by reduction in the length of alkyl chain (n = 10 to 9), emerged as most potent inhibitor with IC50 = 0.68, 24.3, 0.024 μM against PvNMT, PfNMT, LdNMT, respectively. The compound 274 is reported to be the most active inhibitor against L. donovani NMT till date with lower activity against HsNMT1 (IC50 = 60 nM). The SAR has shed light on the importance of N-terminal moiety of peptidomimetic which is expected to be involved in strong electrostatic interaction with C-terminal carboxylate leucine of the enzyme which is crucial for activation of N-terminal amine of peptide substrate. Although these inhibitors display moderate selectivity against human NMT, the sequence alignment of residues as well as the structures of these inhibitors can facilitate a structure-guided design of more potent and selective parasitic inhibitors.158
6. Cytosol
Cytosol is the location of various enzymes involved in numerous metabolic pathways which are essential for parasite growth and propagation in both of its host, female Anopheles mosquito and human being. It has been reported that due to the evolutionarily well conserved pathways, many of these parasite and host targets are quite similar that pose challenges to the design and development of inhibitors that selectively target parasite cytosolic enzymes.26 The discussion of the inhibitors targeting particular enzymes involved in various pathways operating in cytosol such as folate metabolism, glycolysis, purine salvage and pyrimidine synthetic pathway etc. is given in this section:Shahinas et al. explored one of the most potent representatives of purine scaffold inhibitors, PU-H71 (275) as an antimalarial agent (Fig. 33). The compound exhibited high binding affinity to the PfHsp90 ATP-binding domain with Kd of 70.8 μM in comparison to GA derivative 17-AAG, 276 as positive control with Kd of 105 μM and it was suggested that Arg98 guanidinium group plays a key role in accommodating 275 in the PfHsp90 ATP binding pocket. It also inhibited ATPase activity with an IC50 value of 511 nM while 276 displayed IC50 value of 146 nM. Compound 275 showed potent antiparasitic effect in cell culture with IC50 value of 111 nM as well as in the P. berghei in vivo mouse model of malaria. The synergistic effect of 275 shown with chloroquine displayed increase in activity. Based on biochemical and genetic studies, the group postulated that a direct association existed between PfHsp90 and P. falciparum chloroquine resistance transporter (PfCRT) which is responsible for the mechanism of synergism. The synergism between 275 and chloroquine helped in reducing parasite load and improvement in the survival rate in the P. berghei BALB/c mouse model. The PU-H71 (275) and chloroquine is an appealing combination therapy to be used against malarial parasite.167
Hsp90 from P. falciparum were characterised by estimating its ATPase activity as well as drug (geldanamycin), 276 binding abilities and assessed its potential as an antimalarial drug target in mouse model. The studies demonstrated the highest ATPase activity of purified full length PfHsp90 among all known Hsp90s and it exhibited 6 fold higher ATPase activity than that of human Hsp90. Compound 276 showed vigorous inhibition of PfHsp90 ATPase activity (IC50 = 207 nM) in comparison to human Hsp90 (IC50 = 702 nM). Another derivative of GA, 17-(allylamino)-17-demethoxygeldanamycin, 277 showed efficacy in inhibiting parasite growth and increasing the survival rate in the mouse model of malaria. Both 276 and the derivative, 277 were also found to be effective against clinical resistant strains. Compounds 276 and 277 displayed IC50 values of 20 nM and 150 nM for complicated (RK1), respectively while they showed IC50 value of 5.0 nM and 50 nM for uncomplicated (DL1) malaria, after 48 h of treatment. The effect of GA and its derivative in inhibiting T. evansi growth was also evaluated which potentially inhibit T. evansi growth in the mouse model of trypanosomiasis. This biochemical characterization, drug interaction and in vivo studies advocated Hsp90 to be exploited as drug target and the potential of its inhibitors as antiprotozoal agents.166
A robotic high throughput screen (HTS) on 4000 small molecules from three different libraries comprising natural compounds, pharmacologically active scaffolds and FDA approved drugs was evaluated for the competitive inhibitions of ATP-binding domain of PfHsp90. Hits were further evaluated for the specific competitive inhibition of ATP-binding domain of PfHsp90 over that of human Hsp90. Three compounds, (±)-2-amino-3-phosphonopropionic acid, 278 harmine, 279 acrisorcin, 280 were identified which exhibited ≥70% reduction in fluorescence for PfHsp90 while no significant reduction was observed for HsHsp90 (Fig. 33). When evaluated in the cell based antimalarial assay, all the three compounds displayed IC50 values in the nanomolar range against 3D7, W2 as well as multi-drug resistant clinical isolate 208432. The three compounds also demonstrated synergistic activity with chloroquine. This combination strategy is beneficial in counteracting the resistance associated with available antimalarial drugs. The substantial antimalarial effect and synergism with antimalarial agents make these compounds attractive candidate for further clinical development.164
In another study, the group further studied the selectivity of harmine, 279 for PfHsp90 ATP-binding pocket based on surface plasmon resonance studies. It also carried out synergistic activity of 279 with other antimalarials like artemisinin which showed an average ƩFIC50 of 0.1 for harmine and artemisinin, with the use of 3D7. In vivo synergistic data presented a combination of 75 mg kg−1 harmine and 5 mg kg−1 chloroquine as the lowest dose for achieving the synergistic activity which resulted in an average of 97.8% inhibition of parasitemia with no significant toxic effect. These results are encouraging for the development of novel chemotherapeutic combinations that are synergistic with existing antimalarials.168
Wang et al. exploited both the static and dynamic differences between the human and Plasmodium Hsp90s so as to develop pathogen-selective inhibitors. The exploration of the ATP-binding pocket showed that Plasmodium possesses a specific extension in the ATP-binding pocket whose sequence lining is identical to human Hsp90 but differ in their tertiary structure and dynamics. This understanding regarding the structure-based drug screen led to the discovery of 7-azaindole derivatives 281, 282 and 283 that possess the ability to selectively bind to the recombinant N-terminal domain of PfHsp90 over that of human as well as PfHsp90 mutant with “human-like” dynamics. Furthermore, these compounds flaunted the preferential inhibition of the growth of yeast complemented by PfHsp90 and blockage of the growth of Plasmodium in culture.165
Molecular docking studies were performed to identify potential inhibitors of PfLDH by using Molegro Virtual Docker software to analyse potential binding of selected compounds in the active site of PfLDH. Selected compounds with the best binding energies: itraconazole, 284 atorvastatin, 285 and posaconazole, 286 were evaluated for their antiparasitic effect against P. falciparum chloroquine-resistant blood parasites (Fig. 34). All the three compounds exhibited potency in the immunoenzymatic assay carried out using monoclonals specific to PfLDH or histidine rich protein (HRP2). Among three drugs tested, 286 showed lowest IC50 value of 2.6 and 5.3 μM, respectively but was 40- and 100-folds less active than chloroquine in both PfLDH and (HRP2) assays, respectively. These compounds were also found to be effective in reducing parasitemia in P. berghei treated mice. These results well supported the molecular docking analysis confirming it to be an important strategy for the discovery of new antimalarial drugs.169
Ethnopharmacological guided screening was done along with structure-based drug discovery approach for the identification of traditional herbs which caused specific inhibition of Plasmodium LDH. Effect of 8 plants in four different solvents was evaluated on the purified and recombinant PfLDH and PvLDH and aqueous extract of Phyllanthus amarus and chloroform extract of Murraya koenigii exhibited highest potency on rPfLDH (IC50 = 11.2 μg ml−1) and rPvLDH (IC50 = 6.0 μg ml−1), respectively without exerting any inhibitory effect on bovine heart and bovine muscle LDH. Aqueous extract of Phyllanthus amarus displayed its antiparasitic potential on the P. falciparum NE (chloroquine sensitive) and MRC-2 (chloroquine resistant) strains (IC50 = 7.1 μg ml−1 and 6.9 μg ml−1, respectively). This type of study is beneficial and economical over high throughput screening, thus presenting an efficient approach for antimalarial drug discovery programmes.170
Sethiya et al. investigated the inhibitory effect of methanolic extract of Evolvulus alsinoids Linn. (E. alsinoids) on PfLDH. The extract was characterized by thin layer chromatography which led to the identification of one marker compound, scopoletin, 287 and caused reduction in the PfLDH activity (25.04%).171 Bioactivity-guided isolation of antiplasmodial constituents from Conyza sumatrensis (Retz.) E. H. Walker (Cs) leaves which is used for the malaria treatment in Cameroon. The methanolic extract of C. sumatrensis exhibited IC50 value of 36 μg ml−1 which was fractionated into n-hexane, chloroform, ethyl acetate, n-butanol and water fractions. Antiplasmodial evaluation of these fractions revealed chloroform fraction to be most active (IC50, 20 μg ml−1) followed by n-hexane (IC50, 36 μg ml−1), ethyl acetate (IC50, 44 μg ml−1), n-butanol (IC50, 86 μg ml−1) and water (IC50, >100 μg ml−1) fractions. The chromatographic separation of the hexane fraction led to the isolation of three homogeneous compounds: (2S)-1,2-di-O-[(9Z)-octadeca-9-enoyl]-3-O-β-D-galactopyranosyl glycerol (288) (IC50, 34 μg ml−1); (2S)-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-3-O-(6-sulpho-α-D) quinovopyranosyl glycerol (289) (IC50, 17.9 μg ml−1) and 1-O-β-D-glucopyranosyl-(2S,3R,8E)-2-[(2′R)-2-hydroxy-palmitoylamino]-8-octadecene-1,3-diol (290) (IC50, 18 ± 0.12 μg ml−1). Similarly, one homogeneous compound was obtained from the ethyl acetate fraction: 3-O-β-D-glucopyranosyl-3,4-dihydroxybenzoic acid (291) (IC50, 25 μg ml−1). Treatment of P. berghei K-173 infected mouse with these fractions showed chloroform fraction to be most effective in lowering parasitemia followed by n-butanol and ethylacetate fractions. These isolated constituents did not induce any toxicity to murine macrophages and erythrocytes. Compounds 289, 290 and 291 exhibited the inhibition of LDH with IC50 values of 21, 39 and 42 μg ml−1, respectively.172
Based on the observation that uridine can act as substrate for PfPNP, Cui et al. synthesized two series of inhibitors where series one was based on human uridine phosphorylase (UP) inhibitors while series 2 comprised of uracil based analogues to mimic the PNP transition state. Enzyme inhibition results showed that human UP inhibitors were ineffective against PfPNP while uracil based transition state analogues exhibited substantial inhibition with Ki in the micromolar range with the most active one, 292 having Ki value of 6.0 μM. Unfortunately, these compounds even the most active ones did not exert any significant antiparasitic effect which may be due to the low cellular permeability as indicated by low log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P.173
P.173
A series of 8-aryl and 8-pyridylinosines by direct arylation reaction at position 8 of purine nucleosides was also reported. Hydrolytic stability of these compounds was studied at different pH values and inhibitory potential was assessed against P. falciparum PNP. Cytotoxic evaluation for these compounds against different types of cell cultures indicating no significant toxicity which supported the absence of metal-contaminants. Enzymatic evaluation against PfPNP revealed compounds 293, 294 and 295 inhibited the enzyme in the low micromolar range Ki values (Ki = 8.1, 3.7 and 5.3 μM, respectively) which is similar to that of Km of inosine (Km = 5.9 μM) (Fig. 35). Compounds did not exert any inhibitory or substrate activity against human and prokaryotic PNP suggesting 8-arylinosines to be selective inhibitors of PfPNP. Regrettably, lack of significant inhibitory activity against growth of intraerythrocytic forms of the P. falciparum chloroquine-sensitive strain 3D7 hampered its suitability to be used as antimalarial agent. But the enzymatic potency associated with it makes it an appealing starting point to be developed further as specific inhibitors of the enzyme.175
Lautre et al. synthesized Zn(II), Co(II) and Cr(II) complexes of 2-amino-5-nitro-N-hydroxybenzamidine (296, 297 and 298, respectively) and evaluated them for the inhibition of PfPNP and growth of P. falciparum. The ligands as well as their metal complexes displayed good to excellent activity in which 297 showed the highest activity with EC50 of 65 μM (87% inhibition) and 298 (EC50 = 70 μM (85% inhibition)), 296 (EC50 = 85 μM (67% inhibition)) were also active in growth assay. These ligand and complexes were evaluated for their PfPNP inhibition potential and found that compound 297 with Ki of 20 μM (IC50 = 65 μM) was most effective in the enzyme inhibition assay. The Ki (IC50) values for 296 and 298 were 35 (85) μM and 29 (70) μM, respectively. Thus, such type of compounds can be exploited further for their multi-target effects.176
A series of first generation small molecule dual inhibitors of FP-2 and DHFR based on lead compound 299 bearing sulfamide and amide moieties was randomly identified by screening FP-2 inhibitors (Fig. 37). Six compounds reportedly showed improved dual inhibitory activities as compared to lead compound 299 while the inhibitory potential of compound 300 containing thiazole group on amide moiety against FP-2 (IC50 = 7.0 μM) and DHFR (IC50 = 6.3 μM) were increased ∼8 and ∼6 times than that of compound 299, respectively. Moreover, compound 300 displayed moderate in vivo antimalarial activity in a dose dependent fashion, its safety and survival rate were slightly better than that of positive control, chloroquine diphosphate salt. Thus, these dual inhibitors may provide alternate ‘combination therapy’ by linking two pharmacophores in a single structure thereby avoiding the pharmacokinetic disadvantages of two separate agents.180
Adane et al. reported potent Plasmodium DHFR inhibitors based on guanylthiourea scaffold, synthesized mainly from the guanylthiourea and alkyl bromides. In vitro evaluation demonstrated that two compounds, 301 and 302 were potent with the IC50 value of 100 μM and 400 nM, respectively (Fig. 37). The enzyme inhibition data clearly showed the importance of meta-substitution with a bulky atom and bisubstituted guanylthiourea compound also exhibited good activity. This enhanced activity might be due to increase in the hydrogen bond interactions in the active site of PfDHFR enzyme. Thus, the mono-substituted and bi-substituted guanylthiourea derivatives can be explored further in order to improve the activity of this class of compounds.181
Yuthavong et al. reported development of highly selective and efficacious drug candidate to preclinical stage based on the co-crystal structures with inhibitors and substrates along with efficacy and pharmacokinetic profiling. Due to low bioavailability and gastrointestinal toxicity associated with triazine based drug, 2,4-diaminopyrimidine scaffold was chosen for further chemical exploration and this strategy led to the development of 303 which was found to be low nanomolar range inhibitor of wild-type and quadruple mutant PfDHFR as well as exhibited superior oral bioavailability in comparison to triazine based drugs. To increase the tighter binding and DHFR selectivity, fourteen molecules were synthesized and among them, 304 emerged as the most potent compound which was synthesized by replacing trichlorophenyl group of 303 by 2′-carboxyethylphenyl group. Compound 304 showed slow-off tight binding to the wild-type and quadruple mutant DHFR in the subnanomolar range. It exhibited potency against PYR-resistant P. falciparum with quadruple mutant PfDHFR in vitro. In vivo studies against P. chabaudi in CD-1 mice and against quadruple mutant P. falciparum in SCID mice (ED50 = 0.3 mg kg−1, ED90 = 1 mg kg−1) indicated high potency. Compound 304 is now in preclinical trial, the inhibitor carboxylate group showed H-bond interaction with Arg122 of PfDHFR-TS (Fig. 36). Thus, the factors such as good pharmacokinetic profile in preliminary studies in mice and rats, good oral bioavailability (46%), reasonable half-life, safety profile presented 304 as a suitable candidate for further development.182
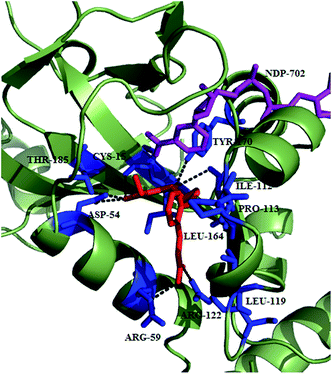 | ||
| Fig. 36 Binding of 304 in the cavity of PfDHFR-TS. Protein is shown in green colour which is making hydrogen bond interactions with 304 (red in colour). The image was created in PyMol using the PDB ID: 4DP3. | ||
The enzymatic activity of PfCA in terms of CO2 hydrase activity showed that PfCA exhibited good CO2 hydrase activity at pH 7.5 with a kcat of 1.4 × 105 s−1 and a kcat/KM of 5.4 × 106 M−1 × s−1. Carbonic anhydrase inhibition potential for inorganic and complex anions and other molecules known to bind with zinc proteins was also studied and it was found that bicarbonate (HCO3−) 305, carbonate (CO32−) 306, nitrate (NO3−) 307, and selenite (SeO4−) 308, were effective micromolar inhibitor of PfCA (Ki = 0.66–0.90 mM) while less active against human isoforms I and II (Fig. 38). The most potent PfCA inhibitors were sulfamide (H2NSO2NH2) 309, sulfamic acid (H2NSO3H), 310, phenylboronic acid (Ph-B(OH)2), 311 and phenylarsonic acid (Ph-AsO3H2) 312, which exhibited inhibition constants in the low micromolar range with Ki of 6.0–9.0 μM. The phylogenetic analysis and a deep investigation of sequence of PfCA and other CAs from other Plasmodium species described that these protozoa encode for new genetic family of CAs designated as η-CA class which is elucidated in the report.186
Inhibition studies of the recently discovered Plasmodium η-CA from Plasmodium against a series of sulfonamides and sulfamates were performed. The potent inhibitors found were ethoxzolamide, 313 and sulthuame, 314 (Ki of 131–132 nM), followed by acetazolamide, 315 methazolamide, 316 and hydrochlorothiazide, 317 with Ki of 153–198 nM. Brinzolamide, 318 topiramate, 319 zonisamide, 320 indisulam, 321 valdecoxib, 322 and celecoxib, 323 also showed substantial inhibitory action against PfCA, with Ki in the range of 217–308 nM. The potent PfCA inhibitors identified so far can be arranged into a variety of chemical classes, ranging from aromatic primary sulfonamides, to monocyclic/bicyclic and heterocyclic derivatives on the basis of inhibition data. Thus, these chemical classes can be further explored to develop efficient, low nanomolar range inhibitors.187
A library of aromatic/heterocyclic sulfonamides bearing various scaffolds were evaluated bearing a wide variety of scaffolds against PfCA enzyme and in vitro P. falciparum growth as well as in vivo efficacy. Among all the compounds, 4-(3,4-dichlorophenylureido)thioureido-benzenesulfonamide, 324 was most potent PfCA inhibitor (Ki of 0.18 μM) with highest potency exhibited in vitro P. falciparum growth inhibition with IC50 of 1.0 μM. In vivo assay in P. berghei mouse model demonstrated its efficacy with ID50 of 10 mg kg−1 in comparison to chloroquine with ID50 of 5 mg kg−1. Thus, these potential sulphonamides can be explored further for the development of novel chemotherapeutic agents against malaria.183
About 133 structurally varied natural compounds from the MEGx® collection of AnalytiCon Discovery and three synthetic hispolone derivatives were screened for their binding potential against Plasmodium thioredoxin reductase (PfTrxR) utilizing ultrafiltration (UF) and liquid chromatography (LC/MS) based ligand-binding assay. Nine compounds (yohimbine (325), catharanthine (326), vobasine (327), gnetifolin E (328), quinidine N-oxide (329), 11-hydroxycoronaridine (330), hispolone (331), hispolone methyl ether (332), and hernagine (333)) exhibited binding to the PfTrxR at 1.0 μM and their binding affinities ranked in the order as 331 > 330 > 326 > 328 > 329 > 332 > 325 > 333 > 327 when tested individual (Fig. 39). When they were tested in an equimolar mixture of 1.0 μM conc., compounds 330, 331, 326 and 338 displayed specific binding to the active site of PfTXR. The highest binding of 331 may be attributed to the presence of hydroxyl groups on the phenyl ring which were supposed to be involved in the hydrogen bonding to cysteine residue of the active site.188
LC/MS spectroscopy and ultrafiltration techniques were used for screening detannified methanolic extracts from Guatteria recurvisepala, Licania kallunkiae and Topobea watsonii to identify natural products for PfTrxR inhibition. The % inhibition of Plasmodium growth of these plant extracts was found to be 57%, 86% and 58%, respectively. When incubated with 1.0 μM PfTrxR, the extract of Guatteria recurvisepala displayed a relative binding affinity of 3.5-fold to the enzyme. The potential compound in the extract was identified as oleamide, 334 based on MS/MS experiments which exhibited in vitro antiparasitic effect against K1 strain with IC50 value of 4.29 μg ml−1.191
A LC-MS based functional assay for evaluating the PfTrxR inhibitory activity showed that out of curcumin, 335 demethoxycurcumin, 336 and bis-demethoxycurcumin, 337 inhibited PfTrxR with IC50 value of 2.0 μM (Fig. 39). Antiplasmodial effect of 335 and 336 showed activity against the chloroquine sensitive D6 strain of P. falciparum and moderate activity against the chloroquine resistant W2 strain. Their cytotoxicity against Vero cells was found to be 64 μM and selectivity index of 4–6 folds and 1-fold against D6 and W2 respectively.192
Theobald et al. discovered and characterized seven molecules identified from an antimalarial set of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 compounds via single-target TrxR biochemical assays. They also produced highly pure recombinant, full-length, untagged enzymes from P. falciparum, P. vivax, P. berghei, and P. yoelii as well as Trx substrates from P. falciparum and R. norvegicus. The identified compounds exhibited specificity for PfTrxR and the cytotoxicity <20% at 10 μM. Mechanism of action of these compounds, 338, 339 was found to be non-competitive in nature with respect to both substrates, with Ki values ranging from 1.3–4.1 μM. Non-competitive inhibition suggested that the inhibitor binds to the enzyme probably in the intersubunit region which is outside the ‘substrates binding sites’.190
533 compounds via single-target TrxR biochemical assays. They also produced highly pure recombinant, full-length, untagged enzymes from P. falciparum, P. vivax, P. berghei, and P. yoelii as well as Trx substrates from P. falciparum and R. norvegicus. The identified compounds exhibited specificity for PfTrxR and the cytotoxicity <20% at 10 μM. Mechanism of action of these compounds, 338, 339 was found to be non-competitive in nature with respect to both substrates, with Ki values ranging from 1.3–4.1 μM. Non-competitive inhibition suggested that the inhibitor binds to the enzyme probably in the intersubunit region which is outside the ‘substrates binding sites’.190
Aculeatin-like analogues were designed and synthesised by combining two sets of building blocks, many of which were active against P. falciparum whole cell assay. SAR around spirocyclohexadienone pharmacophoric scaffold revealed the preference of aliphatic long chain with optimized lipophilicity at position R5 and benzyl group. The presence of aculeatin-like scaffold was found to be of utmost importance in augmenting the antimalarial activity. The most active compounds in this series were 340 and 341 with the same appendages at R4 and R5 position having IC50 values of 1.35 and 2.29 μM, respectively. Among the selected compounds, compound 340 was slightly more selective against P. falciparum over human cell lines such as lymphoblast Jurkat and erythroblast K562 strains. Furthermore, a new aculeatin-like scaffold, 342 lacking Michael acceptor properties was identified, found active at 0.86 μM against P. falciparum 3D7 strain with selectivity index over 30. Enzyme inhibition data for these active compounds showed that (−)-aculeatin A completely blocked the transfer of electrons from PfTrxR to the macromolecular acceptor Trx. They dramatically reducted the electron transfer from NADPH to the FAD active site by 68%, thus 32% residual activity was observed while compounds 340 and 342 exhibited 33% and 74% residual activity, respectively. These results strongly emphasized on the involvement of other plasmodial targets for the strong antiplasmodial activity of 342 that raises its potential to be developed as antimalarial agents with wide druggable spectrum.193
Series of α-/β-thymidine analogues were designed, synthesised and evaluated for PfTMK inhibition based on the report that a series of 5′-substituted α-thymidine derivatives have displayed submicromolar inhibition against MtTMK and selectivity over hTMK. Both the 5′-urea-α- and β-thymidine derivatives exhibited moderate inhibition of the enzyme (Ki = 50–200 μM) as well as moderate antiparasitic effect. The most active α-thymidine inhibitors among the series were 3-trifluoromethyl-4-chlorophenyl derivative 343 (Ki = 31 μM) and the 4-nitrophenyl derivative 344 (Ki = 11 μM) while β-thymidine inhibitors, 3-trifluoromethyl-4-chlorophenyl derivatives 345 (Ki = 25 μM) and 346 (Ki = 27 μM) and the 4-nitrophenyl derivative 347 (Ki = 11 μM) showed slightly better efficacy in enzyme inhibition (Fig. 41). Despite poor enzyme inhibition, some of the derivatives exhibited potent antimalarial effect suggesting a different mode of action of these compounds. Optimisation of the 5′-para substituted phenyl urea α-thymidine derivatives identified a compound, 348 with potent antimalarial activity with selectivity over human ortholog (EC50 = 28 nM; CC50 = 29 μM). SAR analysis revealed the higher potency of α-anomers than β-anomers as well as preference for para substitution. Hydrophobic substituents at para position as well as presence of urea linker showed increased growth inhibition. In vitro DMPK studies on selected compounds showed reasonable microsomal stability in presence of hepatic microsomes except compound 348 which showed rapid turnover. Crystal structure of PfTMK in complex with 345 revealed that thymidine ring mimics the natural TMP substrate. Deoxyribose ring acquires such orientation which allows thymidine to have maximum interactions within the binding pocket of the enzyme (Fig. 40).194
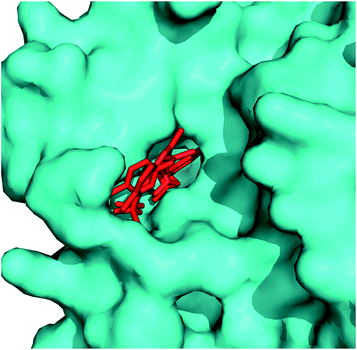 | ||
| Fig. 40 Surface diagram of PfTMK showing 345 (red colour) in the binding pocket. PyMol was used to draw the structure using PDB ID: 2YOF. | ||
Inhibitory potential of seven carbocyclic analogues was screened against PfTMK. The inhibitory results showed that 2′,3′-dideoxycarbocyclic derivative of thymidine, ±349 was the most potent inhibitor. When TMP and dGMP were used as substrate, it exhibited Ki value of 20 μM and 7.0 μM, respectively. All the tested compounds displayed 3–4 fold more potency against deoxyguanylate kinase than the thymidylate kinase, indicating that thymidine derivatives were proficient of competing with guanylate for its active site. When compared with inhibition potential of other analogues, SAR studies suggested the preference for the 2′-deoxy or 2′,3′-dideoxy carbocyclic derivatives over the 2′,3′-dihydroxy derivatives. Since the inhibition was found in low micromolar range, further modifications are needed to produce more potent PfTMK inhibitor.195
In order to improve the inhibitory activity of 2′,3′-dideoxycarbocyclic thymidine derivatives, Noguchi et al. synthesized enantioselective 2′,3′-dideoxycarbocyclic pyrimidine nucleosides and screened them for inhibitory activity against PfTMK. The most potent inhibitor fluorinated dideoxy derivative (−)-350 exhibited KTMPi and KdGMPi values of 14 and 20 μM, respectively as compared to the parent compound (±-351) (KTMPi and KdGMPi values of 20 and 7.0 μM, respectively). Further modifications of (−)-350 will produce much efficient inhibitors thus, presenting an effective strategy to develop safe and selective inhibitors.197
Conclusion and future prospects
Increasing resistance to available malaria chemotherapeutics prompted the search of potential antimalarials that could overcome the shortcomings of the existing therapies. The whole genome sequence of P. falciparum has emerged as a powerful weapon for the discovery of new targets that helped in designing targeted inhibitors. Structure based drug design approach could provide the insight details of structure of the target protein, compound binding and inhibition mechanism. In this extensive review, we have discussed available key targets essential for the parasite survival and growth and their inhibitors derived from plant secondary metabolites, their modifications and synthetic routes following structure-guided drug discovery approach. It is conceivable that other (yet unknown) targets will be disclosed in near future which can be exploited by medicinal chemists for discovery and development of new drugs against malaria.Conflicts of interest
Authors declare no competing interests.Abbreviations
| AMA1 | Apical membrane antigen 1 |
| CA | Carbonic anhydrase |
| CDK | Cyclin dependent protein kinase |
| CPMG | Carr–Purcell–Meiboom–Gill |
| dGDP | Deoxyguanosine monophosphate |
| dGMP | Deoxyguanosine monophosphate |
| DHFR | Dihydrofolate reductase |
| DHODH | Dihydroorotate dehydrogenase |
| DHPS | Dihydropteroate synthase |
| DMAPP | Dimethylallyl diphosphate |
| DPAP | Dipeptidyl aminopeptidase |
| DXR | 1-Deoxy-D-xylulose-5-phosphate reductoisomerase |
| DXP | 1-Deoxy-D-xylulose 5-phosphate |
| FabG | β-Ketoacyl-ACP reductase |
| FabI/ENR | Enoyl-ACP reductase |
| FabZ | β-Hydroxyacyl-ACP dehydratase |
| FAS-II | Fatty acid synthesis type II |
| FMN | Flavin mononucleotide |
| FP | Falcipain |
| HAP | Histo-aspartic protease |
| hCatD | Human cathepsin D |
| HDAC | Histone deacetylase |
| Hsp 90 | Heat shock protein 90 |
| IPP | Isopentenyl diphosphate |
| LDH | Lactate dehydrogenase |
| LE | Ligand efficiency |
| MAP | Metalloaminopeptidases |
| M1AAP | M1 alanyl aminopeptidase |
| M17LAP | M17 leucine aminopeptidase |
| MEP | 2-Methyl-D-erythritol-4-phosphate |
| MQO | Malate:quinone oxidoreductase |
| MSP | Merozoite surface proteins |
| NDH2 | NADH:ubiquinone oxidoreductase |
| NMT | N-Myristoyltransferase |
| PM | Plasmepsin |
| PNP | Purine nucleoside phosphorylase |
| PV | Parasitophorous vacuole |
| RBC | Red blood cells |
| RON | Rhoptry neck protein |
| SAR | Structure–activity relationship |
| SDH | Succinate:ubiquinone oxidoreductase |
| SPR | Surface plasmon resonance |
| STD | Saturation transfer difference |
| SUB1 | Subtilisin-like protease 1 |
| TDP | Thymidine diphosphate |
| TMK | Thymidylate kinase |
| TMP | Thymidine monophosphate |
| TrxR | Thioredoxin reductase |
| TS | Thymidylate synthase |
| WHO | World Health Organization |
| ZBG | Zinc binding group |
Acknowledgements
Mohammad Abid gratefully acknowledges the funding support in the form of Young Scientist from Science & Engineering Research Board (Grant No. SR/FT/LS-03/2011) and University Grant Commission (UGC), (Grant No. 41-277/2012 (SR)), New Delhi, Govt. of India. BA and BK acknowledge the fellowship support from UGC, INDIA.References
- U. R. Mane, D. Mohanakrishnan, D. Sahal, P. R. Murumkar, R. Giridhar and M. R. Yadav, Eur. J. Med. Chem., 2014, 79, 422–435 CrossRef CAS PubMed.
- WHO Report, 2014. http://apps.who.int/iris/bitstream/10665/144852/2/9789241564830_eng.pdf.
- N. Boonyalai, P. Sittikul and J. Yuvaniyama, Mol. Biochem. Parasitol., 2015, 201, 5–15 CrossRef CAS PubMed.
- P. F. Salas, C. Herrmann and C. Orvig, Chem. Rev., 2013, 113, 3450–3492 CrossRef CAS PubMed.
- D. A. Fidock, P. J. Rosenthal, S. L. Croft, R. Brun and S. Nwaka, Nat. Rev. Drug Discovery, 2004, 3, 509–520 CrossRef CAS PubMed.
- R. Gaur, M. P. Darokar, P. V. Ajayakumar, R. S. Shukla and R. S. Bhakuni, Phytochemistry, 2014, 107, 135–140 CrossRef CAS PubMed.
- B. Dali, M. Keita, E. Megnassan, V. Frecer and S. Miertus, Chem. Biol. Drug Des., 2012, 79, 411–430 CAS.
- P. J. Rosenthal, J. Exp. Biol., 2003, 206, 3735–3744 CrossRef CAS PubMed.
- J. Held, A. Kreidenweiss and B. Mordmüller, Expert Opin. Drug Discovery, 2013, 8, 1325–1337 CrossRef CAS PubMed.
- N. Drinkwater and S. McGowan, Biochem. J., 2014, 461, 349–369 CrossRef CAS PubMed.
- E. Y. Klein, Int. J. Antimicrob. Agents, 2013, 41, 311–317 CrossRef CAS PubMed.
- J. Valbuena, L. Rodríguez, R. Vera, A. Puentes, H. Curtidor, J. Cortés, J. Rosas and M. E. Patarroyo, Biochimie, 2006, 88, 1447–1455 CrossRef CAS PubMed.
- S. S. Lim, C. O. Debono, C. A. MacRaild, I. R. Chandrashekaran, O. Dolezal, R. F. Anders, J. S. Simpson, M. J. Scanlon, S. M. Devine, P. J. Scammells and R. S. Norton, Aust. J. Chem., 2013, 66, 1530–1536 CrossRef CAS.
- A. Alam, M. Goyal, M. S. Iqbal, C. Pal, S. Dey, S. Bindu, P. Maity and U. Bandyopadhyay, Expert Rev. Clin. Pharmacol., 2009, 2, 469–489 CrossRef CAS PubMed.
- S. M. Devine, S. S. Lim, I. R. Chandrashekaran, C. A. MacRaild, D. R. Drew, C. O. Debono, R. Lam, R. F. Anders, J. G. Beeson, M. J. Scanlon, P. J. Scammells and R. S. Norton, Med. Chem. Commun., 2014, 5, 1500–1506 RSC.
- E. F. Lee, S. Yao, J. K. Sabo, W. D. Fairlie, R. A. Stevenson, K. S. Harris, R. F. Anders, M. Foley and R. S. Norton, Biopolymers, 2011, 95, 354–364 CrossRef CAS PubMed.
- B. V. Normand, M. L. Tonkin, M. H. Lamarque, S. Langer, S. Hoos, M. Roques, F. A. Saul, B. W. Faber, G. A. Bentley, M. J. Boulanger and M. Lebrun, PLoS Pathog., 2012, 8, e1002755 Search PubMed.
- A. Alam, Malar. Res. Treat., 2014, 20–22 Search PubMed.
- P. Srinivasan, A. Yasgar, D. K. Luci, W. L. Beatty, X. Hu, J. Andersen, D. L. Narum, J. K. Moch, H. Sun, J. D. Haynes, D. J. Maloney, A. Jadhav, A. Simeonov and L. H. Miller, Nat. Commun., 2013, 4, 2261 Search PubMed.
- S. Giovani, M. Penzo, S. Brogi, M. Brindisi, S. Gemma, E. Novellino, L. Savini, M. J. Blackman, G. Campiani and S. Butini, Bioorg. Med. Chem. Lett., 2014, 24, 3582–3586 CrossRef CAS PubMed.
- S. Giovani, M. Penzo, S. Butini, M. Brindisi, S. Gemma, E. Novellino, G. Campiani, M. J. Blackman and S. Brogi, RSC Adv., 2015, 5, 22431–22448 RSC.
- C. Withers-Martinez, C. Suarez, S. Fulle, S. Kher, M. Penzo, J. Ebejer, K. Koussis, F. Hackett, A. Jirgensons, P. Finn and M. J. Blackman, Int. J. Parasitol., 2012, 42, 597–612 CrossRef CAS PubMed.
- C. Withers-Martinez, M. Strath, F. Hackett, L. F. Haire, S. A. Howell, P. A. Walker, E. Christodoulou, G. G. Dodson and M. J. Blackman, Nat. Commun., 2014, 5, 3726 CAS.
- S. Gemma, S. Giovani, M. Brindisi, P. Tripaldi, S. Brogi, L. Savini, I. Fiorini, E. Novellino, S. Butini, G. Campiani, M. Penzo and M. J. Blackman, Bioorg. Med. Chem. Lett., 2012, 22, 5317–5321 CrossRef CAS PubMed.
- S. S. Kher, M. Penzo, S. Fulle, P. W. Finn, M. J. Blackman and A. Jirgensons, Bioorg. Med. Chem. Lett., 2014, 24, 4486–4489 CrossRef CAS PubMed.
- P. J. Rosenthal, J. Exp. Biol., 2003, 206, 3735–3744 CrossRef CAS PubMed.
- T. S. Skinner-Adams, C. M. Stack, K. R. Trenholme, C. L. Brown, J. Grembecka, J. Lowther, A. Mucha, M. Drag, P. Kafarski, S. McGowan, J. C. Whisstock, D. L. Gardiner and J. P. Dalton, Trends Biochem. Sci., 2009, 35, 53–61 CrossRef PubMed.
- Z. S. Saify, M. K. Azim, W. Ahmad, M. Nisa, D. E. Goldberg, S. A. Hussain, S. Akhtar, A. Akram, A. Arayne, A. Oksman and I. A. Khan, Bioorg. Med. Chem. Lett., 2012, 22, 1282–1286 CrossRef CAS PubMed.
- Y. Song, H. Jin, X. Liu, L. Zhu, J. Huang and H. Li, Bioorg. Med. Chem. Lett., 2013, 23, 2078–2082 CrossRef CAS PubMed.
- S. C. Mohapatra, H. K. Tiwari, M. Singla, B. Rathi, A. Sharma, K. Mahiya, M. Kumar, S. Sinha and S. S. Chauhan, J. Biol. Inorg. Chem., 2010, 15, 373–385 CrossRef CAS PubMed.
- T. Luksch, A. Blum, N. Klee, W. E. Diederich, C. A. Sotriffer and G. Klebe, ChemMedChem, 2010, 5, 443–454 CrossRef CAS PubMed.
- W. Ahmed, M. Rani, I. A. Khan, A. Iqbal, K. M. Khan, M. A. Haleem and M. K. Azim, J. Enzyme Inhib. Med. Chem., 2010, 25, 673–678 CrossRef CAS PubMed.
- D. Gupta, R. S. Yedidi, S. Varghese, L. C. Kovari and P. M. Woster, J. Med. Chem., 2010, 53, 4234–4247 CrossRef CAS PubMed.
- Z. S. Saify, M. Nisa, K. F. Azhar, M. K. Azim, H. Rasheed, N. Mushtaq, M. A. Arain, S. Haider, M. Khanum and W. Ahmed, Nat. Prod. Res., 2011, 25, 1965–1968 CrossRef CAS PubMed.
- N. Vaiana, M. Marzahn, S. Parapini, P. Liu, M. Dell'Agli, A. Pancotti, E. Sangiovanni, N. Basilico, E. Bosisio, B. M. Dunn, D. Taramelli and S. Romeo, Bioorg. Med. Chem. Lett., 2012, 22, 5915–5918 CrossRef CAS PubMed.
- V. Aureggi, V. Ehmke, J. Wieland, W. B. Schweizer, B. Bernet, D. Bur, S. Meyer, M. Rottmann, C. Freymond, R. Brun, B. Breit and F. Diederich, Chem.–Eur. J., 2013, 19, 155–164 CrossRef CAS PubMed.
- M. J. Meyers, M. D. Tortorella, J. Xu, L. Qin, Z. He, X. Lang, W. Zeng, W. Xu, L. Qin, M. J. Prinsen, F. M. Sverdrup, C. S. Eickhoff, D. W. Griggs, J. Oliva, P. G. Ruminski, E. J. Jacobsen, M. A. Campbell, D. C. Wood, D. E. Goldberg, X. Liu, Y. Lu, X. Lu, Z. Tu, X. Lu, K. Ding and X. Chen, ACS Med. Chem. Lett., 2014, 5, 89–93 CrossRef CAS PubMed.
- K. Jaudzems, K. Tars, G. Maurops, N. Ivdra, M. Otikovs, J. Leitans, I. Kanepe-Lapsa, I. Domraceva, I. Mutule, P. Trapencieris, M. J. Blackman and A. Jirgensons, ACS Med. Chem. Lett., 2014, 5, 373–377 CrossRef CAS PubMed.
- H. Jin, Z. Xu, K. Cui, T. Zhang, W. Lu and J. Huang, Fitoterapia, 2014, 94, 55–61 CrossRef CAS PubMed.
- A. Nezami, I. Luque, T. Kimura, Y. Kiso and E. Freire, Biochemistry, 2002, 41, 2273–2280 CrossRef CAS PubMed.
- A. Kiso, K. Hidaka, T. Kimura, Y. Hayashi, A. Nezami, E. Freire and Y. Kiso, J. Pept. Sci., 2004, 10, 641–647 CrossRef CAS PubMed.
- T. Miura, K. Hidaka, T. Uemura, K. Kashimoto, Y. Hori, Y. Kawasaki, A. J. Ruben, E. Freire, T. Kimura and Y. Kiso, Bioorg. Med. Chem. Lett., 2010, 20, 4836–4839 CrossRef CAS PubMed.
- T. Miura, K. Hidaka, Y. Azai, K. Kashimoto, Y. Kawasaki, S. Chen, R. F. de Freitas, E. Freire and Y. Kiso, Bioorg. Med. Chem. Lett., 2014, 24, 1698–1701 CrossRef CAS PubMed.
- A. Mahindra, R. P. Gangwal, S. Bansal, N. E. Goldfarb, B. M. Dunn, A. T. Sangamwar and R. Jain, RSC Adv., 2015, 5, 22674–22684 RSC.
- J. M. Coterón, D. Catterick, J. Castro, M. J. Chaparro, B. Diaz, E. Fernández, S. Ferrer, F. J. Gamo, M. Gordo, J. Gut, L. de las Heras, J. Legac, M. Marco, J. Miguel, V. Muñoz, E. Porras, J. C. de la Rosa, J. R. Ruiz, E. Sandoval, P. Ventosa, P. J. Rosanthal and J. M. Fiandor, J. Med. Chem., 2010, 53, 6129–6152 CrossRef PubMed.
- R. Ettari, A. Pinto, L. Tamborini, I. C. Angelo, S. Grasso, M. Zappalà, N. Capodicasa, L. Yzeiraj, E. Gruber, M. N. Aminake, G. Pradel, T. Schirmeister, C. De Micheli and P. Conti, ChemMedChem, 2014, 9, 1817–1825 CAS.
- B. Rathi, A. K. Singh, R. Kishan, N. Singh, N. Latha, S. Srinivasan, K. C. Pandey, H. K. Tiwari and B. K. Singh, Bioorg. Med. Chem., 2013, 21, 5503–5509 CrossRef CAS PubMed.
- R. Löser, J. Gut, P. J. Rosenthal, M. Frizler, M. Gütschow and K. T. Andrews, Bioorg. Med. Chem. Lett., 2010, 20, 252–255 CrossRef PubMed.
- L. Rizzi, S. Sundararaman, K. Cendic, N. Vaiana, R. Korde, D. Sinha, A. Mohmmed, P. Malhotra and S. Romeo, Eur. J. Med. Chem., 2011, 46, 2083–2090 CrossRef CAS PubMed.
- B. Somanadhan, S. R. Kotturi, C. Y. Leong, R. P. Glover, Y. Huang, H. Flotow, A. D. Buss, M. J. Lear and M. S. Butler, J. Antibiot., 2013, 66, 259–264 CrossRef CAS PubMed.
- T. Conroy, J. T. Guo, N. Elias, K. M. Cergol, J. Gut, J. Legac, L. Khatoon, Y. Liu, S. McGowan, P. J. Rosenthal, N. H. Hunt and R. J. Payne, J. Med. Chem., 2014, 57, 10557–10563 CrossRef CAS PubMed.
- S. K. Chakka, M. Kalamuddin, S. Sundararaman, L. Wei, S. Mundra, R. Mahesh, P. Malhotra, A. Mohmmed and L. P. Kotra, Bioorg. Med. Chem., 2015, 23, 2221–2240 CrossRef CAS PubMed.
- F. Bova, R. Ettari, N. Micale, C. Carnovale, T. Schirmeister, C. Gelhaus, M. Leippe, S. Grasso and M. Zappalà, Bioorg. Med. Chem., 2010, 18, 4928–4938 CrossRef CAS PubMed.
- V. Ehmke, F. Kilchmann, C. Heindl, K. Cui, J. Huang, T. Schirmeister and F. Diederich, Med. Chem. Commun., 2011, 2, 800–804 RSC.
- R. Ettari, M. Zappalà, N. Micale, T. Schirmeister, C. Gelhaus, M. Leippe, A. Evers and S. Grasso, Eur. J. Med. Chem., 2010, 45, 3228–3233 CrossRef CAS PubMed.
- D. J. Weldon, F. Shah, A. G. Chittiboyina, A. Sheri, R. R. Chada, J. Gut, P. J. Rosenthal, D. Shivakumar, W. Sherman, P. Desai, J. Jung and M. A. Avery, Bioorg. Med. Chem. Lett., 2014, 24, 1274–1279 CrossRef CAS PubMed.
- Y. Liu, W. Lu, K. Cui, W. Luo, J. Wang and C. Guo, Arch. Pharmacal Res., 2012, 35, 1525–1531 CrossRef CAS PubMed.
- Y. Liu, K. Cui, W. Lu, W. Luo, J. Wang, J. Huang and C. Guo, Molecules, 2011, 16, 4527–4538 CrossRef CAS PubMed.
- W. Luo, W. Lu, K. Cui, Y. Liu, J. Wang and C. Guo, Med. Chem. Res., 2012, 21, 3073–3079 CrossRef CAS.
- E. M. Guantai, K. Ncokazi, T. J. Egan, J. Gut, P. J. Rosenthal, P. J. Smith and K. Chibale, Bioorg. Med. Chem., 2010, 18, 8243–8256 CrossRef CAS PubMed.
- E. M. Guantai, K. Ncokazi, T. J. Egan, J. Gut, P. J. Rosenthal, R. Bhampidipati, A. Kopinathan, P. J. Smith and K. Chibale, J. Med. Chem., 2011, 54, 3637–3649 CrossRef CAS PubMed.
- B. Pérez, C. Teixeira, J. Gut, P. J. Rosenthal, J. R. B. Gomes and P. Gomes, ChemMedChem, 2012, 7, 1537–1540 CrossRef PubMed.
- M. Akhter, R. Saha, O. Tanwar, M. M. Alam and M. S. Zaman, Med. Chem. Res., 2014, 24, 879–890 CrossRef.
- T. Waag, C. Gelhaus, J. Rath, A. Stich, M. Leippe and T. Schirmeister, Bioorg. Med. Chem. Lett., 2010, 20, 5541–5543 CrossRef CAS PubMed.
- L. Wang, H. He, Y. Di, Y. Zhang and X. Hao, Tetrahedron Lett., 2012, 53, 1576–1578 CrossRef CAS.
- S. C. Stolze, E. Deu, F. Kaschani, N. Li, B. I. Florea, K. H. Richau, T. Colby, R. A. L. van der Hoorn, H. S. Overkleeft, M. Bogyo and M. Kaiser, Chem. Biol., 2012, 19, 1546–1555 CrossRef CAS PubMed.
- E. Salas-Sarduy, A. Cabrera-Muñoz, A. Cauerhff, Y. González-González, S. A. Trejo, A. Chidichimo, M. de los Angeles Chávez-Planes and J. J. Cazzulo, Exp. Parasitol., 2013, 135, 611–622 CrossRef CAS PubMed.
- L. Wang, S. Zhang, J. Zhu, L. Zhu, X. Liu, L. Shan, J. Huang, W. Zhang and H. Li, Bioorg. Med. Chem. Lett., 2014, 24, 1261–1264 CrossRef CAS PubMed.
- R. G. Kamkumo, A. M. Ngoutane, L. R. Y. Tchokouaha, P. V. T. Fokou, E. A. K. Madiesse, J. Legac, J. J. B. Kezetas, B. N. Lenta, F. F. Boyom, T. Dimo, W. F. Mbacham, J. Gut and P. J. Rosenthal, Malar. J., 2012, 11, 382–389 CrossRef PubMed.
- R. Oliveira, R. C. Guedes, P. Miereles, I. S. Albuquerque, L. M. Gonçalves, E. Pires, M. R. Bronze, J. Gut, P. J. Rosenthal, M. Prudêncio, R. Moreira, P. M. O'Neill and F. Lopes, J. Med. Chem., 2014, 57, 4916–4923 CrossRef CAS PubMed.
- U. R. Mane, H. Li, J. Huang, R. C. Gupta, S. S. Nadkarni, R. Giridhar, P. P. Naik and M. R. Yadav, Bioorg. Med. Chem., 2012, 20, 6296–6304 CrossRef CAS PubMed.
- R. H. Hans, J. Gut, P. J. Rosenthal and K. Chibale, Bioorg. Med. Chem. Lett., 2010, 20, 2234–2237 CrossRef CAS PubMed.
- N. Tadigoppula, V. Korthikunta, S. Gupta, P. Kancharla, T. Khaliq, A. Soni, R. K. Srivastava, K. Srivastava, S. K. Puri, K. S. R. Raju, Wahajuddin, P. S. Sijwali, V. Kumar and I. S. Mohammad, J. Med. Chem., 2013, 56, 31–45 CrossRef CAS PubMed.
- J. B. Bertolodo, L. D. Chiaradia-Deatorre, A. Mascarello, P. C. Leal, M. N. S. Cordeiro, R. J. Nunes, E. S. Sarduy, P. J. Rosenthal and H. Terenzi, J. Enzyme Inhib. Med. Chem., 2014, 30, 299–307 CrossRef PubMed.
- R. Mahesh, S. Mundra, T. Devadoss and L. P. Kotra, Arabian J. Chem., 2014 DOI:10.1016/j.arabjc.2014.11.008.
- R. Ettari, L. Tamborini, I. C. Angelo, S. Grasso, T. Schirmeister, L. Lo Presti, C. De Micheli, A. Pinto and P. Conti, ChemMedChem, 2013, 8, 2070–2076 CrossRef CAS PubMed.
- R. K. Sharma, Y. Younis, G. Mugumbate, M. Njoroge, J. Gut, P. J. Rosenthal and K. Chibale, Eur. J. Med. Chem., 2015, 90, 507–518 CrossRef CAS PubMed.
- V. Ehmke, C. Heindl, M. Rottmann, C. Freymond, W. B. Schweizer, R. Brun, A. Stich, T. Schirmeister and F. Diederich, ChemMedChem, 2011, 6, 273–278 CrossRef CAS PubMed.
- S. D. Khanye, G. S. Smith, C. Lategan, P. J. Smith, J. Gut, P. J. Rosenthal and K. Chibale, J. Inorg. Biochem., 2010, 104, 1079–1083 CrossRef CAS PubMed.
- S. Langolf, U. Machon, M. Ehlers, W. Sicking, T. Schirmeister, C. Büchhold, C. Gelhaus, P. J. Rosenthal and C. Schmuck, ChemMedChem, 2011, 6, 1581–1586 CrossRef CAS PubMed.
- N. Micale, M. A. Cinellu, L. Maiore, A. R. Sannella, C. Severini, T. Schirmeister, C. Gabbiani and L. Messori, J. Inorg. Biochem., 2011, 105, 1576–1579 CrossRef CAS PubMed.
- F. Shah, Y. Wu, J. Gut, Y. Pedduri, J. Legac, P. J. Rosenthal and M. A. Avery, Med. Chem. Commun., 2011, 2, 1201–1207 RSC.
- S. P. Kumar, J. Gut, R. C. Guedes, P. J. Rosenthal, M. M. M. Santos and R. Moreira, Eur. J. Med. Chem., 2011, 46, 927–933 CrossRef PubMed.
- R. Oliveira, A. S. Newton, R. C. Guedes, D. Miranda, R. K. Amewu, A. Srivastava, J. Gut, P. J. Rosenthal, P. M. O'Neill, S. A. Ward, F. Lopes and R. Moreira, ChemMedChem, 2013, 8, 1528–1536 CrossRef CAS PubMed.
- P. Gibbons, E. Verissimo, N. C. Araujo, V. Barton, G. L. Nixon, R. K. Amewu, J. Chadwick, P. A. Stocks, G. A. Biagini, A. Srivastava, P. J. Rosenthal, J. Gut, R. C. Guedes, R. Moreira, R. Sharma, N. Berry, M. L. S. Cristiano, A. E. Shone, S. A. Ward and P. M. O'Neill, J. Med. Chem., 2010, 53, 8202–8206 CrossRef CAS PubMed.
- A. K. Singh, V. Rajendran, A. Pant, P. C. Ghosh, N. Singh, N. Latha, S. Garg, K. C. Pandey, B. K. Singh and B. Rathi, Bioorg. Med. Chem., 2015, 23, 1817–1827 CrossRef CAS PubMed.
- S. N. Mistry, N. Drinkwater, C. Ruggeri, K. K. Sivaraman, S. Loganathan, S. Fletcher, M. Drag, A. Paiardini, V. M. Avery, P. J. Scammells and S. McGowan, J. Med. Chem., 2014, 57, 9168–9183 CrossRef CAS PubMed.
- K. K. Sivaraman, A. Paiardini, M. Siénczyk, C. Ruggeri, C. A. Oellig, J. P. Dalton, P. J. Scammells, M. Drag and S. McGowan, J. Med. Chem., 2013, 56, 5213–5217 CrossRef CAS PubMed.
- G. Velmourougane, M. B. Harbut, S. Dalal, S. McGowan, C. A. Oellig, N. Meinhardt, J. C. Whisstock, M. Klemba and D. C. Greenbaum, J. Med. Chem., 2011, 54, 1655–1666 CrossRef CAS PubMed.
- T. S. Skinner-Adams, C. L. Peatey, K. Anderson, K. R. Trenholme, D. Krige, C. L. Brown, C. Stack, D. M. M. Nsangou, R. T. Mathews, K. Thivierge, J. P. Dalton and D. L. Gardiner, Antimicrob. Agents Chemother., 2012, 56, 3244–3249 CrossRef CAS PubMed.
- R. Deprez-Poulain, M. Flipo, C. Piveteau, F. Leroux, S. Dassonneville, I. Florent, L. Maes, P. Cos and B. Deprez, J. Med. Chem., 2012, 55, 10909–10917 CrossRef CAS PubMed.
- T. Spicer, V. Fernandez-Vega, P. Chase, L. Scampavia, J. To, J. P. Dalton, F. L. Da Silva, T. S. Skinner-Adams, D. L. Gardiner, K. R. Trenholme, C. L. Brown, P. Ghosh, P. Porubsky, J. L. Wang, D. A. Whipple, F. J. Schoenen and P. Hodder, J. Biomol. Screening, 2014, 19, 1107–1115 CrossRef CAS PubMed.
- A. Paiardini, R. S. Bamert, K. Kannan-Sivaraman, N. Drinkwater, S. N. Mistry, P. J. Scammells and S. McGowen, PLoS One, 2015, 2, e0115859 Search PubMed.
- F. Wang, P. Krai, E. Deu, B. Bibb, C. Lauritzen, J. Pedersen, M. Bogyo and M. Klemba, Mol. Biochem. Parasitol., 2011, 175, 10–20 CrossRef CAS PubMed.
- E. Deu, M. J. Leyva, V. E. Albrow, M. J. Rice, J. A. Ellman and M. Bogyo, Chem. Biol., 2010, 17, 808–819 CrossRef CAS PubMed.
- N. C. Wheatley, K. T. Andrews, T. L. Tran, A. J. Lucke, R. C. Reid and D. P. Fairlie, Bioorg. Med. Chem. Lett., 2010, 20, 7080–7084 CrossRef CAS PubMed.
- F. K. Hansen, T. S. Skinner-Adams, S. Duffy, L. Marek, S. D. M. Sumanadasa, K. Kuna, J. Held, V. M. Avery, K. T. Andrews and T. Kurz, ChemMedChem, 2014, 9, 665–670 CrossRef CAS PubMed.
- V. Patil, W. Guerrant, P. C. Chen, B. Gryder, D. B. Benicewicz, S. I. Khan, B. L. Tekwani and A. K. Oyelere, Bioorg. Med. Chem., 2010, 18, 415–425 CrossRef CAS PubMed.
- S. D. M. Sumanadasa, C. D. Goodman, A. J. Lucke, T. Skinner-Adams, I. Sahama, A. Haque, T. A. Do, G. I. McFadden, D. P. Fairlie and K. T. Andrews, Antimicrob. Agents Chemother., 2012, 56, 3849–3856 CrossRef CAS PubMed.
- F. K. Hansen, S. D. M. Sumanadasa, K. Stenzel, S. Duffy, S. Meister, L. Marek, R. Schmetter, K. Kuna, A. Hamacher, B. Mordmüller, M. U. Kassack, E. A. Winzeler, V. M. Avery, K. T. Andrews and T. Kurz, Eur. J. Med. Chem., 2014, 82, 204–213 CrossRef CAS PubMed.
- G. Giannini, G. Battistuzzi and D. Vignola, Bioorg. Med. Chem. Lett., 2015, 25, 459–461 CrossRef CAS PubMed.
- J. A. Geyer, S. M. Keenan, C. L. Woodard, P. A. Thompson, L. Gerena, D. A. Nichols, C. E. Gutteridge and N. C. Waters, Bioorg. Med. Chem. Lett., 2009, 19, 1982–1985 CrossRef CAS PubMed.
- C. L. Woodard, S. M. Keenan, L. Gerena, W. J. Welsh, J. A. Geyer and N. C. Waters, Bioorg. Med. Chem. Lett., 2007, 17, 4961–4966 CrossRef CAS PubMed.
- D. Caridha, A. K. Kathcart, D. Jirage and N. C. Waters, Bioorg. Med. Chem. Lett., 2010, 20, 3863–3867 CrossRef CAS PubMed.
- P. A. Stocks, V. Barton, T. Antoine, G. A. Biagini, S. A. Ward and P. M. O'Neill, Parasitology, 2013, 141, 50–65 CrossRef PubMed.
- J. M. Bueno, E. Herreros, I. Angulo-Barturen, S. Ferrer, J. M. Fiandor, F. J. Gamo, D. Gargallo-Viola and G. Derimanov, Future Med. Chem., 2012, 4, 2311–2323 CrossRef CAS PubMed.
- G. L. Nixon, C. Pidathala, A. E. Shone, T. Antoine, N. Fisher, S. A. Ward, P. M. O'Neill, S. A. Ward and G. A. Biagini, Future Med. Chem., 2013, 5, 1573–1591 CrossRef CAS PubMed.
- R. Cowley, S. Leung, N. Fisher, M. Al-Helal, N. G. Berry, A. S. Lawrenson, R. Sharma, A. E. Shone, S. A. Ward, G. A. Biagini and P. M. O'Neill, Med. Chem. Commun., 2012, 3, 39–44 RSC.
- M. J. Capper, P. M. O'Neill, N. Fisher, R. W. Strange, D. Moss, S. A. Ward, N. G. Berry, A. S. Lawrenson, S. S. Hasnain, G. A. Biagini and S. V. Antonyuk, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 755–760 CrossRef CAS PubMed.
- C. Vallières, N. Fisher, T. Antoine, M. Al-Helal, P. Stocks, N. G. Berry, A. S. Lawrenson, S. A. Ward, P. M. O'Neill, G. A. Biagini and B. Meunier, Antimicrob. Agents Chemother., 2012, 56, 3739–3747 CrossRef PubMed.
- P. Zhao, L. Wang, X. Zhu, X. Huang, C. Zhan, J. Wu and G. Yang, J. Am. Chem. Soc., 2010, 132, 185–194 CrossRef CAS PubMed.
- N. Kongkathip, N. Pradidphol, K. Hasitapan, R. Grigg, W. Kao, C. Hunte, N. Fisher, A. J. Warman, G. A. Biagini, P. Kongsaeree, P. Chuawong and B. Kongkathip, J. Med. Chem., 2010, 53, 1211–1221 CrossRef CAS PubMed.
- A. Nilsen, G. P. Miley, I. P. Forquer, M. W. Mather, K. Katneni, Y. Li, S. Pou, A. M. Pershing, A. M. Stickles, E. Ryan, J. X. Kelly, J. S. Doggett, K. L. White, D. J. Hinrichs, R. W. Winter, S. A. Charman, L. N. Zakharov, I. Bathurst, J. N. Burrows, A. B. Vaidya and M. K. Riscoe, J. Med. Chem., 2014, 57, 3818–3834 CrossRef CAS PubMed.
- D. C. Schuck, S. B. Ferreira, L. N. Cruz, D. R. da Rocha, M. S. Moraes, M. Nakabashi, P. J. Rosenthal, V. F. Ferreira and C. R. S. Garcia, Malar. J., 2013, 234, 1–6 Search PubMed.
- C. K. Dong, S. Urgaonkar, J. F. Cortese, F. Gamo, J. F. Garcia-Bustos, M. J. Lafuente, V. Patel, L. Ross, B. I. Coleman, E. R. Derbyshire, C. B. Clish, A. E. Serrano, M. Cromwell, R. H. Barker, J. D. Dvorin, M. T. Duraisingh, D. F. Wirth, J. Clardy and R. Mazitschek, Chem. Biol., 2011, 18, 1602–1610 CrossRef CAS PubMed.
- L. M. Hughes, C. A. Lanteri, M. T. O'Neil, J. D. Johnson, G. W. Gribble and B. L. Trumpower, Mol. Biochem. Parasitol., 2011, 177, 12–19 CrossRef CAS PubMed.
- S. C. Teguh, N. Klonis, S. Duffy, L. Lucantoni, V. M. Avery, C. A. Hutton, J. B. Baell and L. Tilley, J. Med. Chem., 2013, 56, 6200–6215 CrossRef CAS PubMed.
- T. Nam, C. W. McNamara, S. Bopp, N. V. Dharia, S. Meister, G. M. C. Bonamy, D. M. Plouffe, N. Kato, S. McCormack, B. Bursulaya, H. Ke, A. B. Vaidya, P. G. Schultz and E. A. Winzeler, ACS Chem. Biol., 2011, 6, 1214–1222 CrossRef CAS PubMed.
- F. P. da Cruz, C. Martin, K. Buchholz, M. J. Lafuente-Monasterio, T. Rodrigues, B. Sönnichsen, R. Moreira, F. J. Gamo, M. Marti, M. M. Mota, M. Hannus and M. Prudêncio, J. Infect. Dis., 2012, 205, 1278–1286 CrossRef CAS PubMed.
- X. Deng, D. Matthews, P. K. Rathod and M. A. Phillips, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2015, 71, 553–559 CrossRef CAS PubMed.
- J. M. Coteron, M. Marco, J. Esquivias, X. Deng, K. L. White, J. White, M. Koltun, F. El Mazouni, S. Kokkonda, K. Katneni, R. Bhamidipati, D. M. Shackleford, I. Angulo-Barturen, S. B. Ferrer, M. B. Jiménez-Díaz, F. Gamo, E. J. Goldsmith, W. N. Charman, I. Bathurst, D. Floyd, D. Matthews, J. N. Burrows, P. K. Rathod, S. A. Charman and M. A. Phillips, J. Med. Chem., 2011, 54, 5540–5561 CrossRef CAS PubMed.
- P. K. Ojha, I. Mitra, S. Kar, R. N. Das and K. Roy, Mol. Inf., 2012, 31, 711–718 CrossRef.
- D. Cowen, P. Bedingfield, G. A. McConkey, C. W. G. Fishwick and A. P. Johnson, Bioorg. Med. Chem. Lett., 2010, 20, 1284–1287 CrossRef CAS PubMed.
- M. L. Booker, C. M. Bastos, M. L. Kramer, R. H. Barker, R. Skerlj, A. B. Sidhu, X. Deng, C. Celatka, J. F. Cortese, J. E. G. Bravo, K. N. C. Llado, A. E. Serrano, I. Angulo-Barturen, M. B. Jiménez-Diaz, S. Viera, H. Garuti, S. Wittlin, P. Papastogiannidis, J. Lin, C. J. Janse, S. M. Khan, M. Duraisingh, B. Coleman, E. J. Goldsmith, M. A. Phillips, B. Munoz, D. F. Wirth, J. D. Klinger, R. Wiegand and E. Sybertz, J. Biol. Chem., 2010, 285, 33054–33064 CrossRef CAS PubMed.
- R. Gujjar, A. Marwaha, F. El Mazouni, J. White, K. L. White, S. Creason, D. M. Shackleford, J. Baldwin, W. N. Charman, F. S. Buckner, S. Charman, P. K. Rathod and M. A. Phillips, J. Med. Chem., 2009, 52, 1864–1872 CrossRef CAS PubMed.
- R. Gujjar, F. El Mazouni, K. L. White, J. White, S. Creason, D. M. Shackleford, X. Deng, W. N. Charman, I. Bathurst, J. Burrows, D. M. Floyd, D. Matthews, F. S. Buckner, S. A. Charman, M. A. Phillips and P. K. Rathod, J. Med. Chem., 2011, 54, 3935–3949 CrossRef CAS PubMed.
- R. T. Skerlj, C. M. Bastos, M. L. Booker, M. L. Kramer, R. H. Barker Jr, C. A. Celatka, T. J. O'Shea, B. Munoz, A. B. Sidhu, J. F. Cortese, S. Wittlin, P. Papastogiannidis, I. Angulo-Barturen, A. B. Jimenez-Diaz and E. Sybertz, ACS Med. Chem. Lett., 2011, 2, 708–713 CrossRef CAS PubMed.
- I. Fritzson, P. T. P. Bedingfield, A. P. Sundin, G. McConkey and U. J. Nilsson, Med. Chem. Commun., 2011, 2, 895–898 RSC.
- A. Marwaha, J. White, F. El Mazouni, S. A. Creason, S. Kokkonda, F. S. Buckner, S. A. Charman, M. A. Phillips and P. K. Rathod, J. Med. Chem., 2012, 55, 7425–7436 CrossRef CAS PubMed.
- M. Xu, J. Zhu, Y. Diao, H. Zhou, X. Ren, D. Sun, J. Huang, D. Han, Z. Zhao, L. Zhu, Y. Xu and H. Li, J. Med. Chem., 2013, 56, 7911–7924 CrossRef CAS PubMed.
- G. A. Biagini, N. Fisher, A. E. Shone, M. A. Mubaraki, A. Srivastava, A. Hill, T. Antoine, A. J. Warman, J. Davies, C. Pidathala, R. K. Amewu, S. C. Leung, R. Sharma, P. Gibbons, D. W. Hong, B. Pacorel, A. S. Lawrenson, S. Chareonsutthivarakul, L. Taylor, O. Berger, A. Mbekeani, P. A. Stocks, G. L. Nixon, J. Chadwick, J. Hemingway, M. J. Delves, R. E. Sinden, A. Zeeman, C. H. M. Kocken, N. G. Berry, P. M. O'Neill and S. A. Ward, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 8298–8303 CrossRef CAS PubMed.
- C. K. Dong, V. Patel, J. C. Yang, J. D. Dvorin, M. T. Duraisingh, J. Clardy and D. F. Wirth, Bioorg. Med. Chem. Lett., 2009, 19, 972–975 CrossRef CAS PubMed.
- C. Pidathala, R. Amewu, B. Pacorel, G. L. Nixon, P. Gibbons, W. D. Hong, S. C. Leung, N. G. Berry, R. Sharma, P. A. Stocks, A. Srivastava, A. E. Shone, S. Charoensutthivarakul, L. Taylor, O. Berger, A. Mbekeani, A. Hill, N. E. Fisher, A. J. Warman, G. A. Biagini, S. A. Ward and P. M. O'Neill, J. Med. Chem., 2012, 55, 1831–1843 CrossRef CAS PubMed.
- S. C. Leung, P. Gibbons, R. Amewu, G. L. Nixon, C. Pidathala, W. D. Hong, B. Pacorel, N. G. Berry, R. Sharma, P. A. Stocks, A. Srivastava, A. E. Shone, S. Charoensutthivarakul, L. Taylor, O. Berger, A. Mbekeani, A. Hill, N. E. Fisher, A. J. Warman, G. A. Biagini, S. A. Ward and P. M. O'Neill, J. Med. Chem., 2012, 55, 1844–1857 CrossRef CAS PubMed.
- N. Arisue and T. Hashimoto, Parasitol. Int., 2015, 64, 254–259 CrossRef PubMed.
- M. J. Shears, C. Y. Botté and G. I. McFadden, Mol. Biochem. Parasitol., 2015, 199, 34–50 CrossRef CAS PubMed.
- I. L. Lauinger, L. Vivas, R. Perozzo, C. Stairiker, A. Tarun, M. Zloh, X. Zhang, H. Xu, P. J. Tonge, S. G. Franzblau, D. Pham, C. V. Esguerra, A. D. Crawford, L. Maes and D. Tasdemir, J. Nat. Prod., 2013, 76, 1064–1070 CrossRef CAS PubMed.
- S. Jensen, S. Omarsdottir, A. G. Bwalya, M. A. Nielsen, D. Tasdemir and E. S. Olafsdottir, Phytomedicine, 2012, 19, 1191–1195 CrossRef CAS PubMed.
- A. K. Gupta, S. Saxena and M. Saxena, Bioorg. Med. Chem. Lett., 2010, 20, 4779–4781 CrossRef CAS PubMed.
- F. Scala, E. Fattorusso, M. Menna, O. Taglialatela-Scafati, M. Tierney, M. Kaiser and D. Tasdemir, Mar. Drugs, 2010, 8, 2162–2174 CrossRef CAS PubMed.
- D. Tasdemir, D. Sanabria, I. L. Lauinger, A. Tarun, R. Herman, R. Perozzo, M. Zloh, S. H. Kappe, R. Brun and N. M. Carballeira, Bioorg. Med. Chem., 2010, 18, 7475–7485 CrossRef CAS PubMed.
- F. Belluti, R. Perozzo, L. Lauciello, F. Colizzi, D. Kostrewa, A. Bisi, S. Gobbi, A. Rampa, M. L. Bolognesi, M. Recanatini, R. Brun, L. Scapozza and A. Cavalli, J. Med. Chem., 2013, 56, 7516–7526 CrossRef CAS PubMed.
- A. Muhammad, I. Anis, Z. Ali, S. Awadelkarim, A. Khan, A. Khalid, M. R. Shah, M. Galal, I. A. Khan and M. I. Choudhary, Bioorg. Med. Chem. Lett., 2012, 22, 610–612 CrossRef CAS PubMed.
- R. P. Samal, V. M. Khedkar, R. R. S. Pissurlenkar, A. G. Bwalya, D. Tasdemir, R. A. Joshi, P. R. Rajamohanan, V. G. Puranik and E. C. Coutinho, Chem. Biol. Drug Des., 2013, 81, 715–729 CAS.
- N. M. Carballeira, A. G. Bwalya, M. A. Itoe, A. D. Andricopulo, M. L. Cordero-Maldonado, M. Kaiser, M. M. Mota, A. D. Crawford, R. V. C. Guido and D. Tasdemir, Bioorg. Med. Chem. Lett., 2014, 24, 4151–4157 CrossRef CAS PubMed.
- L. Tallorin, J. D. Durrant, Q. G. Nguyen, J. A. McCammon and M. D. Burkart, Bioorg. Med. Chem., 2014, 22, 6053–6061 CrossRef CAS PubMed.
- A. S. Murkin, K. A. Manning and S. A. Kholodar, Bioorg. Chem., 2014, 57, 171–185 CrossRef CAS PubMed.
- R. Chofor, S. Sooriyaarachchi, M. D. P. Risseeuw, T. Bergfors, J. Pouyez, C. Johny, A. Haymond, A. Everaert, C. S. Dowd, L. Maes, T. Coenye, A. Alex, R. D. Couch, T. A. Jones, J. Wouters, S. L. Mowbray and S. Van Calenbergh, J. Med. Chem., 2015, 58, 2988–3001 CrossRef CAS PubMed.
- J. Xue, J. Diao, G. Cai, L. Deng, B. Zheng, Y. Yao and Y. Song, ACS Med. Chem. Lett., 2013, 4, 278–282 CrossRef CAS PubMed.
- T. Bodill, A. C. Conibear, M. K. M. Mutorwa, J. L. Goble, G. L. Blatch, K. A. Lobb, R. Klein and P. T. Kaye, Bioorg. Med. Chem., 2013, 21, 4332–4341 CrossRef CAS PubMed.
- C. T. Behrendt, A. Kunfermann, V. Illarionova, A. Matheeussen, T. Gräwert, M. Groll, F. Rohdich, A. Bacher, W. Eisenreich, M. Fischer, L. Maes and T. Kurz, ChemMedChem, 2010, 5, 1673–1676 CrossRef CAS PubMed.
- C. T. Behrendt, A. Kunfermann, V. Illarionova, A. Matheeussen, M. K. Pein, T. Gräwert, J. Kaiser, A. Bacher, W. Eisenreich, B. Illarionov, M. Fischer, L. Maes, M. Groll and T. Kurz, J. Med. Chem., 2011, 54, 6796–6802 CrossRef CAS PubMed.
- K. Brücher, B. Illarionov, J. Held, S. Tschan, A. Kunfermann, M. K. Pein, A. Bacher, T. Gräwert, L. Maes, B. Mordmüller, M. Fischer and T. Kurz, J. Med. Chem., 2012, 55, 6566–6575 CrossRef PubMed.
- A. Kunfermann, C. Lienau, B. Illarionov, J. Held, T. Gräwert, C. T. Behrendt, P. Werner, S. Hähn, W. Eisenreich, U. Riederer, B. Mordmüller, A. Bacher, M. Fischer, M. Groll and T. Kurz, J. Med. Chem., 2013, 56, 8151–8162 CrossRef CAS PubMed.
- S. Konzuch, T. Umeda, J. Held, S. Hähn, K. Brücher, C. Lienau, C. T. Behrendt, T. Gräwert, A. Bacher, B. Illarionov, M. Fischer, B. Mordmüller, N. Tanaka and T. Kurz, J. Med. Chem., 2014, 57, 8827–8838 CrossRef CAS PubMed.
- R. E. Cobb, B. Bae, Z. Li, M. A. DeSieno, S. K. Nair and H. Zhao, Chem. Commun., 2015, 51, 2526–2528 RSC.
- K. Brücher, T. Gräwert, S. Konzuch, J. Held, C. Lienau, C. Behrendt, B. Illarionov, L. Maes, A. Bacher, S. Wittlin, B. Mordmüller, M. Fischer and T. Kurz, J. Med. Chem., 2015, 58, 2025–2035 CrossRef PubMed.
- T. O. Olaleye, J. A. Brannigan, S. M. Roberts, R. J. Leatherbarrow, A. J. Wilkinson and E. W. Tate, Org. Biomol. Chem., 2014, 12, 8132–8137 CAS.
- M. D. Rackham, J. A. Brannigan, K. Rangachari, S. Meister, A. J. Wilkinson, A. A. Holder, R. J. Leatherbarrow and E. W. Tate, J. Med. Chem., 2014, 57, 2773–2788 CrossRef CAS PubMed.
- Z. Yu, J. A. Brannigan, D. K. Moss, A. M. Brzozowski, A. J. Wilkinson, A. A. Holder, E. W. Tate and R. J. Leatherbarrow, J. Med. Chem., 2012, 55, 8879–8890 CrossRef CAS PubMed.
- M. H. Wright, B. Clough, M. D. Rackham, K. Rangachari, J. A. Brannigan, M. Grainger, D. K. Moss, A. R. Bottrill, W. P. Heal, M. Broncel, R. A. Serwa, D. Brady, D. J. Mann, R. J. Leatherbarrow, R. Tewari, A. J. Wilkinson, A. A. Holder and E. W. Tate, Nat. Chem., 2013, 6, 112–121 CrossRef PubMed.
- A. S. Bell, J. E. Mills, G. P. Williams, J. A. Brannigan, A. J. Wilkinson, T. Parkinson, R. J. Leatherbarrow, E. W. Tate, A. A. Holder and D. F. Smith, PLoS Neglected Trop. Dis., 2012, 6, e1625 CAS.
- M. D. Rackham, J. A. Brannigan, D. K. Moss, Z. Yu, A. J. Wilkinson, A. A. Holder, E. W. Tate and R. J. Leatherbarrow, J. Med. Chem., 2013, 56, 371–375 CrossRef CAS PubMed.
- D. Shahinas, M. Liang, A. Datti and D. R. Pillai, J. Med. Chem., 2010, 53, 3552–3557 CrossRef CAS PubMed.
- T. Wang, W. H. Bisson, P. Mäser, L. Scpozza and D. Picard, J. Med. Chem., 2014, 57, 2524–2535 CrossRef CAS PubMed.
- R. Pallavi, N. Roy, R. K. Nageshan, P. Talukdar, S. R. Pavithra, R. Reddy, S. Venketesh, R. Kumar, A. K. Gupta, R. K. Singh, S. C. Yadav and U. Tatu, J. Biol. Chem., 2010, 285, 37964–37975 CrossRef CAS PubMed.
- D. Shahinas, A. Folefoc, T. Taldone, G. Chiosis, I. Crandall and D. R. Pillai, PLoS One, 2013, 8, e75446 CAS.
- D. Shahinas, G. MacMullin, C. Benedict, I. Crandall and D. R. Pillai, Antimicrob. Agents Chemother., 2012, 8, 4207–4213 CrossRef PubMed.
- J. Penna-Coutinho, W. A. Cortopassi, A. A. Oliveira, T. C. C. França and A. U. Krettli, PLoS One, 2011, 6, e21237 CAS.
- P. Keluskar and S. Ingle, J. Ethnopharmacol., 2012, 144, 201–207 CrossRef PubMed.
- N. Sethiya, P. Keluskar, S. Ingle and S. Mishra, Asian Pac. J. Trop. Dis., 2014, 4, 489–491 CrossRef CAS.
- P. K. Boniface, S. Verma, A. Shukla, H. S. Cheema, S. K. Srivastava, F. Khan, M. P. Darokar and A. Pal, Parasitol. Int., 2015, 64, 118–123 CrossRef CAS PubMed.
- H. Cui, G. F. Ruda, J. Carrero-Lérida, L. M. Ruiz-Pérez, I. H. Gilbert and D. González-Pacanowska, Eur. J. Med. Chem., 2010, 45, 5140–5149 CrossRef CAS PubMed.
- T. M. Donaldson, L. Ting, C. Zhan, W. Shi, R. Zheng, S. C. Almo and K. Kim, PLoS One, 2014, 9, e84384 Search PubMed.
- A. Gigante, E. Priego, P. Sánchez-Carrasco, L. M. Ruiz-Pérez, J. V. Voorde, M. Camarasa, J. Balzarini, D. González-Pacanowska and M. Pérez-Pérez, Eur. J. Med. Chem., 2014, 82, 459–465 CrossRef CAS PubMed.
- H. K. Lautre, K. Patil, H. Youssouffi, T. Ben Hadda, V. Bhatia and A. K. Pillai, World J. Pharm. Pharm. Sci., 2014, 3, 1053–1068 CAS.
- J. E. Hyde, Acta Trop., 2005, 94, 191–206 CrossRef CAS PubMed.
- S. Abbat, V. Jain and P. V. Bharatam, J. Biomol. Struct. Dyn., 2014, 1–16 Search PubMed , ahead-of-print.
- C. Capasso and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2013, 29, 379–387 CrossRef PubMed.
- H. Huang, W. Lu, X. Li, X. Cong, H. Ma, X. Liu, Y. Zhang, P. Che, R. Ma, H. Li, X. Shen, H. Jiang, J. Huang and J. Zhu, Bioorg. Med. Chem. Lett., 2012, 22, 958–962 CrossRef CAS PubMed.
- L. Adane, S. Bhagat, M. Arfeen, S. Bhatia, R. Sirawaraporn, W. Sirawaraporn, A. K. Chakraborti and P. V. Bharatam, Bioorg. Med. Chem. Lett., 2014, 24, 613–617 CrossRef CAS PubMed.
- Y. Yuthavong, B. Tarnchompoo, T. Vilaivan, P. Chitnumsub, S. Kamchonwongpaisan, S. A. Charman, D. N. McLennan, K. L. White, L. Vivas, E. Bongard, C. Thongphanchang, S. Taweechai, J. Vanichtankul, R. Rattanajak, U. Arwon, P. Fantauzzi, J. Yuvaniyama, W. N. Charman and D. Matthews, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16823–16828 CrossRef CAS PubMed.
- S. R. Krungkrai and J. Krungkrai, Asian Pac. J. Trop. Biomed., 2011, 1, 233–242 CrossRef CAS PubMed.
- G. De Simone, A. Di Fiore, C. Capasso and C. T. Supuran, Bioorg. Med. Chem. Lett., 2015, 25, 1385–1389 CrossRef CAS PubMed.
- J. Krungkrai, A. Scozzafava, S. Reungprapavut, S. R. Krungkrai, R. Rattanajak, S. Kamchonwongpaisan and C. T. Supuran, Bioorg. Med. Chem., 2005, 13, 483–489 CrossRef CAS PubMed.
- S. D. Prete, D. Vullo, G. M. Fisher, K. T. Andrews, S. Poulsen, C. Capasso and C. T. Supuran, Bioorg. Med. Chem. Lett., 2014, 24, 4389–4396 CrossRef PubMed.
- D. Vullo, S. D. Prete, G. M. Fisher, K. T. Andrews, S. Poulsen, C. Capasso and C. T. Supuran, Bioorg. Med. Chem., 2015, 23, 526–531 CrossRef CAS PubMed.
- R. Munigunti, V. Mulabagal and A. I. Calderón, J. Pharm. Biomed. Anal., 2011, 55, 265–271 CrossRef CAS PubMed.
- R. Munigunti, S. Gathiaka, O. Acevedo, R. Sahu, B. Tekwani and A. I. Calderón, Chem. Cent. J., 2013, 7, 175 CrossRef PubMed.
- A. J. Theobald, I. Caballero, I. Coma, G. Colmenarejo, C. Cid, F. Gamo, M. J. Hibbs, A. L. Bass and D. A. Thomas, Biochemistry, 2012, 51, 4764–4771 CrossRef CAS PubMed.
- R. Munigunti, N. Nelson, V. Mulabagal, M. P. Gupta, R. Brun and A. I. Calderón, Planta Med., 2011, 77, 1749–1753 CrossRef CAS PubMed.
- R. Munigunti, S. Gathiaka, O. Acevedo, R. Sahu, B. Tekwani and A. I. Calderón, Nat. Prod. Res., 2014, 28, 359–364 CrossRef CAS PubMed.
- M. Winkler, M. Maynadier, S. Wein, M. Lespinasse, G. Boumis, A. E. Miele, H. Vial and Y. Wong, Org. Biomol. Chem., 2015, 13, 2064–2077 CAS.
- H. Cui, J. Carrero-Lérida, A. P. G. Silva, J. L. Whittingham, J. A. Brannigan, L. M. Ruiz-Pérez, K. D. Read, K. S. Wilson, D. González-Pacanowska and I. H. Gilbert, J. Med. Chem., 2012, 55, 10948–10957 CrossRef CAS PubMed.
- A. Kato, Y. Yasuda, Y. Kitamura, M. Kandeel and Y. Kitade, Parasitol. Int., 2012, 61, 501–503 CrossRef CAS PubMed.
- P. K. Ojha and K. Roy, BioSystems, 2013, 113, 177–195 CrossRef CAS PubMed.
- Y. Noguchi, Y. Yasuda, M. Tashiro, T. Kataoka, Y. Kitamura, M. Kandeel and Y. Kitade, Parasitol. Int., 2013, 62, 368–371 CrossRef CAS PubMed.
- K. S. Harris, J. L. Casey, A. M. Coley, J. A. Karas, J. K. Sabo, Y. Y. Tan, O. Dolezal, R. S. Norton, A. B. Hughes, D. Scanlon and M. Foley, J. Biol. Chem., 2009, 284, 9361–9371 CrossRef CAS PubMed.
- S. Arastu-Kapur, E. L. Ponder, U. P. Fonović, S. Yeoh, F. Yuan, M. Fonović, M. Grainger, C. I. Phillips, J. C. Powers and M. Bogyo, Nat. Chem. Biol., 2008, 4, 203–213 CrossRef CAS PubMed.
- G. S. Dow, Y. Chen, K. T. Andrews, D. Caridha, L. Gerena, M. Gettayacamin, J. Johnson, Q. Li, V. Melendez, N. Obaldia III, T. N. Tran and A. P. Kozikowski, Antimicrob. Agents Chemother., 2008, 52, 3467–3477 CrossRef CAS PubMed.
- P. Mukherjee, A. Pradhan, F. Shah, B. L. Tekwani and M. A. Avery, Bioorg. Med. Chem., 2008, 16, 5254–5265 CrossRef CAS PubMed.
- C. L. Woodard, Z. Li, A. K. Kathcart, J. Terrell, L. Gerena, M. Lopez-Sanchez, D. E. Kyle, A. K. Bhattacharjee, D. A. Nichola, W. Ellis, S. T. Prigge, J. A. Geyer and N. C. Waters, J. Med. Chem., 2003, 46, 3877–3882 CrossRef CAS PubMed.
- K. Maity, B. S. Venkata, N. Kapoor, N. Surolia, A. Surolia and K. Suguna, J. Struct. Biol., 2011, 176, 238–249 CrossRef CAS PubMed.
- D. Tasdemir, G. Lack, R. Brun, P. Rüedi, L. Scapozza and R. Perozzo, J. Med. Chem., 2006, 49, 3345–3353 CrossRef CAS PubMed.
- R. Conners, F. Schambach, J. Read, A. Cameron, R. B. Sessions, L. Vivas, A. Easton, S. L. Croft and R. L. Brady, Mol. Biochem. Parasitol., 2005, 142, 137–148 CrossRef CAS PubMed.
- A. Cameron, J. Read, R. Tranter, V. J. Winter, R. B. Sessions, R. L. Brady, L. Vivas, A. Easton, H. Kendrick, S. L. Croft, D. Barros, J. L. Lavandera, J. J. Martin, F. Risco, S. G. Ochoa, F. J. Gamo, L. Sanz, L. Leon, J. R. Ruiz, R. Gabarró, A. Mallo and F. G. de Ias Heras, J. Biol. Chem., 2004, 279, 31429–31439 CrossRef CAS PubMed.
- D. C. Madrid, L. Ting, K. L. Waller, V. L. Schramm and K. Kim, J. Biol. Chem., 2008, 283, 35899–35907 CrossRef CAS PubMed.
- J. Yuvaniyama, P. Chitnumsub, S. Kamchonwongpaisan, J. Vanichtanankul, W. Sirawaraporn, P. Taylor, M. D. Walkinshaw and Y. Yuthavong, Nat. Struct. Biol., 2003, 10, 357–365 CrossRef CAS PubMed.
- T. Triglia, J. G. T. Menting, C. Wilson and A. F. Cowman, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 13944–13949 CrossRef CAS.
- L. Jiang, P. Lee, J. White and P. K. Rathod, Antimicrob. Agents Chemother., 2000, 44, 1047–1050 CrossRef CAS PubMed.
- S. Reungprapavut, S. R. Krungkrai and J. Krungkrai, J. Enzyme Inhib. Med. Chem., 2004, 19, 249–256 CrossRef CAS PubMed.
- R. L. Krauth-Siegel and G. H. Coombs, Parasitol. Today, 1999, 15, 404–409 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |