The hierarchical porosity of a three-dimensional graphene electrode for binder-free and high performance supercapacitors†
Abstract
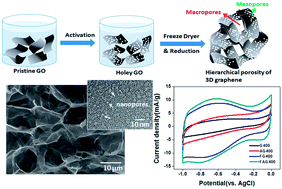
In this study, we report a binder-free supercapacitor including a unique graphene electrode of hierarchical porosity composed of the submicrometer porosity (∼20 μm) of 3D graphene self-assembled by nanopores (∼2.5 nm) of graphene flakes. The hierarchical 3D porosity of the graphene electrode was prepared by a facile and scalable approach based on acid activation and freeze-drying. Remarkably, we found that this unique graphene-based electrode, when used in a supercapacitor, shows improved performance compared with a reported graphene electrode; these improvements include high specific capacitance (∼442 F g−1), fast rate charging/discharging, and excellent cycle stability (95% after 1600 cycle numbers), which were due to (1) the creation of a high specific surface area and a diffusion path, thus promoting ion transport capability, and (2) the additional pseudocapacitor due to the controlled oxygen amount of functionalization on the graphene surface. The resultant device shows an energy density of 51.9 W h kg−1 and a power density of 467.8 W kg−1. This study introduces a new concept for the binder-free, cost-effective and high performance of a graphene-based supercapacitor, which could pave the way for practical applications in frontier energy devices.

 Please wait while we load your content...
Please wait while we load your content...