Qualitative analysis of chiral alanine by UV-visible-shortwave near infrared diffuse reflectance spectroscopy combined with chemometrics
Abstract
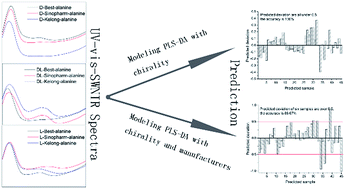
UV-visible-shortwave near infrared diffuse reflectance spectroscopy (UV-vis-SWNIR DRS) combined with chemometrics is investigated for the first time to discriminate enantiomers and their racemic compounds, using D-, L- and DL-alanine as model compounds. After optimizing the measuring conditions of powder particle sizes and the distance between fiber probe and sample, discriminant partial least squares (PLS-DA) models were built with UV-vis-SWNIR DRS to implement the qualitative analysis of alanine chirality. As a result, under the optimized conditions of particle size sifted through 100-mesh and a distance of 5.3 mm, an excellent discrimination of chirality with an accuracy of 100% was obtained, which is better than that of manufacturers with an accuracy of 86.67%. The results of this study infer that UV-vis-SWNIR DRS combined with chemometrics can be a rapid, simple and noninvasive method for chiral analysis.

 Please wait while we load your content...
Please wait while we load your content...