A depropargylation-triggered spontaneous cyclization based fluorescent “turn-on” chemodosimeter for the detection of palladium ions and its application in live-cell imaging†
Abstract
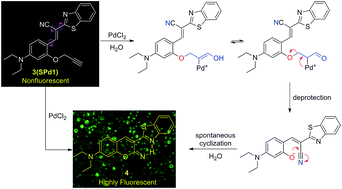
A novel depropargylation-triggered spontaneous cyclization reaction based fluorescent turn-on chemodosimeter for the detection of palladium ions has been rationally designed and developed. Based on the specific reactivity of the palladium promoted hydrolysis reaction, the probe exhibited a high selectivity and sensitivity for palladium ions. Furthermore, the probe was successfully used for fluorescence imaging of Pd2+ in living cells.

 Please wait while we load your content...
Please wait while we load your content...