Theoretical insights into the CO dimerization and trimerization on Pt nanocluster†
Yongpeng Yanga,
Ping Chenga,
Shengli Zhangb and
Shiping Huang*a
aState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: huangsp@mail.buct.edu.cn; Fax: +86-10-64427616
bSchool of Material Science and Technology, Nanjing University of Science and Technology, Nanjing 210094, China
First published on 4th January 2016
Abstract
CO dimerization and trimerization on an icosahedral Pt55 cluster were investigated using density functional theory. It is found that the products of CO polymerization depend on the different active sites of the metal surface and CO coverage. C2O2 can be adsorbed on either two neighboring Pt atoms or one Pt atom, and the former case is preferred. The preference can be ascribed to the stronger interaction between the 8σ orbital of C2O2 and 5d orbitals of Pt in the former case, and this interaction increases the stability of C2O2. Two neighboring adsorbed CO molecules (CO*) can capture one free CO to form a ring-opening CO trimer on the Pt surface. High CO coverage can facilitate the dimerization and trimerization of CO and change the preferred adsorption site of C2O2, and highly-coordinated Pt atoms present the superior chemical activity for CO polymerization at high CO coverage. The CO dimerization by two CO* need to overcome a high energy barrier of 1.92 eV, but one CO* can capture one free CO molecule to form the C2O2 with overcoming a much lower energy barrier of 0.87 eV. The energy barrier of CO trimerization is 1.14 eV.
1. Introduction
Carbon monoxide hydrogenation to form hydrocarbons (Fischer–Tropsch synthesis) has received much attention both in academia and industry with clean transportation fuels produced from natural gas, coal, and biomass, etc.1 The process of Fischer–Tropsch (F–T) synthesis is complex and is composed by several elementary steps: chains initiation, chains growth and chains termination. There are several prevalent mechanisms of CO activation and dissociation in the chains initiation. One of the mechanisms is called H-assisted CO dissociation mechanism, where the chemisorbed hydrogen (H*) bonds with chemisorbed CO molecule (CO*) before the CO dissociation.2–10 The oxygen atoms (O*) in H-assisted CO dissociation are removed as H2O. Another mechanism is called the carbide mechanism, where CO* dissociates directly on the metal surface to form chemisorbed atomic carbon and oxygen species (C* + O*).11–13 Then CHx (x = 1–3) species are formed by the reaction of C* atom and H* atoms, and O* is removed as CO2.1,14–16 In these mechanisms, polymerization of CH2 leads to the growth of chain.17,18However, Schouten et al. postulated a different mechanism of electrochemical reduction of carbon dioxide to ethylene.19 In this mechanism, the first step is the formation of a CO dimer. Although no experiment has directly observed the formation of a CO dimer on a metal surface, theoretical calculations on the C–C coupling provide consistent results with available experimental data. Using DFT, it is found that the CO dimer is preferentially adsorbed on Cu(100) rather than on Cu(111), and H+, Li+ and Na+ can facilitate the C–C coupling.20,21 On the other hand, the mechanism with a rate-determining CO coupling step reveals that on Cu(100) at low overpotential only C2 species can be formed, and the formation of C1 species is hindered.22 These results coincide with the facts found in the experiments, where the formation of C2 species on Cu(100) is found easier than on Cu(111), and on Cu(100) at low overpotential only C2H4 is observed and CH4 is not.23–25 The electrochemical reduction of CO2 can also occur on the other metal surfaces such as Pt, Cu–Au and Pt–Ru/C.26,27 Moreover, C2 species are also found on other metal catalysts such as silver surface in CO2 electrochemical reduction, although the amounts of C2 species are small.28
In fact, C2O2, remaining undetectable even after numerous experimental efforts, is believed to be an intrinsically short-lived molecule. Theoretical calculations indicate C2O2 is a triplet, which indeed rapidly dissociates into two CO molecules with a lifetime of 0.5 ns.29,30 Similarly, neutral (CO)n (n > 2) polymers are also very unstable due to the great tendency toward dissociation into CO molecules.31–34 In order to increase the stability of (CO)n, it has been demonstrated feasible to change (CO)n into positively35,36 or negatively37 charged oxocarbons, which are more stable than the corresponding neutral ones. Low-valent f-elements with high reduction potentials are found to be applicable to CO coupling. Recently, reductive CO coupling, cyclo-trimerization and cyclo-tetramerization at mild conditions have been exhibited by U(III) complexes.38–41 Indeed, head-to-head C–C coupling of six CO molecules was observed in the reduction of CO with a ditantalum hydride complex, and the synthesized (CO)6 is stable unless it is exposed in O2 and H2O.31
In another experiment of CO2 electroreduction on Cu surfaces, C3 species were also detected, and the C–C coupling was presented to be involved in the formation of the C3 species.42 So in this work, the dimerization, as well as the trimerization, of CO is studied to explore the possibility of C–C bond formation before CO dissociation. Fe and Co are the most common catalysts in F–T synthesis industry, but the CO* dissociates directly on these metal surface.13 Important experimental and theoretical studies suggest that the activity for CO dissociation is higher for the metals in left-side of the periodic table than that of the metals on the right-hand side, and it decreases from 3d- to 5d-metals.43,44 The 5d-metals on the right-hand side of the periodic table can be selected to avoid the direct CO dissociation after CO adsorption. Platinum surface may be proper to produce (CO)n because of its low activity of direct CO dissociation, but the activities for other reactions are very high. For instance, H2 can be oxidized by O2 into water on Pt(5 5 7) facet at 298 K.45 Hydrogenation of unsaturated hydrocarbon compounds, as well as pyrrole, is also easy when selecting platinum as catalyst.46 Furthermore, the activities relate to the shape and size of metal, e.g. smaller Pt clusters have higher activities for ethylene hydrogenation.47 High stability of icosahedral Pt55 (∼1 nm) cluster has been demonstrated in many studies.48,49 Thus, we selected the Pt55 cluster to investigate the possible reactions between CO* molecules.
At first, we obtained the most stable geometrical structures of CO, C2O2 and C3O3 adsorbed on the Pt55. The reaction processes of forming C2O2 and C3O3 were carried out with the nudged elastic band (NEB) method.58 Theoretical studies have found CO coverage can dramatically affect the reaction process. CO dissociation on Rh(1 0 0) is hindered when CO coverage is greater than or equal to 3/12 ML,50 and the oxidization of CO* by O2 was facilitated when CO coverage is increased.51 In order to study CO coverage effect, the most stable geometrical structures of C2O2 and C3O3 adsorption, as well as the preferred reaction processes of forming corresponding carbon oxides, were researched with high CO coverage taken into account. Finally, we utilized density of states and Mulliken population analysis to investigate the electronic properties of C2O2 adsorption.
2. Computational details
Spin-polarized density functional theory (DFT) calculations were carried out using the CP2K code52 in the Gaussian and plane waves (GPW) formalism.53 The PBE exchange correlation was employed,54 as well as the DZVP Molopt local basis sets55 in combination with Goedecker–Teter–Hutter (GTH) pseudopotentials56 and a plane wave density cutoff of 300 Ry. The self-consistent field (SCF) convergence criterion was set to be 1.0 × 10−6 Ha and the calculations were performed in a periodic cubic simulation box of 25 × 25 × 25 Å3, large enough to neglect the interaction between the nanoparticles in periodic images. Geometry optimizations were performed using the Broyden–Fletcher–Goldfarb–Shanno (BFGS) minimization algorithm until the maximum atomic force was less than 4.5 × 10−4 Ha per Bohr.57 To compute the reaction barrier we used the climbing image nudged elastic band (CI-NEB) method,58 where the convergence criterion of maximum force on the band was fixed at 2 × 10−3 Ha per Bohr. We chose 8 images for every NEB calculation.The geometrical structure of icosahedral Pt55, as well as one of the (1 1 1) facets, is shown in Fig. S1 in the ESI.† In every facet, there are two different Pt atoms: the corner Pt atom (T1) and the edge Pt atom (T2). The adsorption properties of single CO molecule on Pt55 cluster are investigated to confirm the preferred adsorption site, because it is found that some DFT calculations present inconsistent results compared with many experimental studies at low temperature by underestimating CO preference for low-coordination sites on Pt(111).59 By comparing CO adsorption energies at the six different sites, the adsorption energy decreases in the sequence of T1 > T2 > B1 > B2 > H1 > H2. Namely, the single CO molecule is preferentially adsorbed on the atop site, and the Pt–C bond length is 1.85 Å, which agrees with experimental results fairly well.59–61
3. Results and discussion
3.1. Geometrical structures and reaction processes of CO dimerization and trimerization on Pt55 cluster
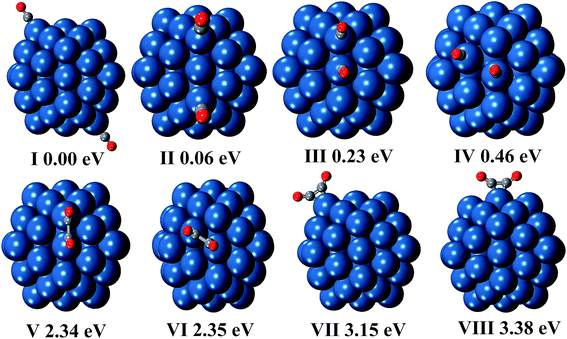
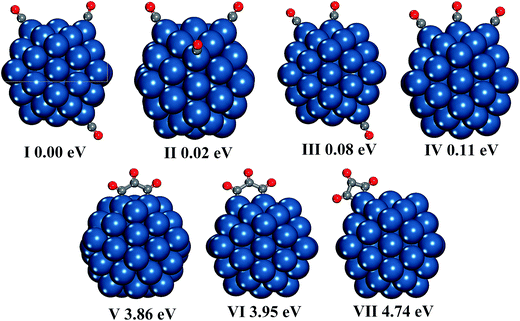
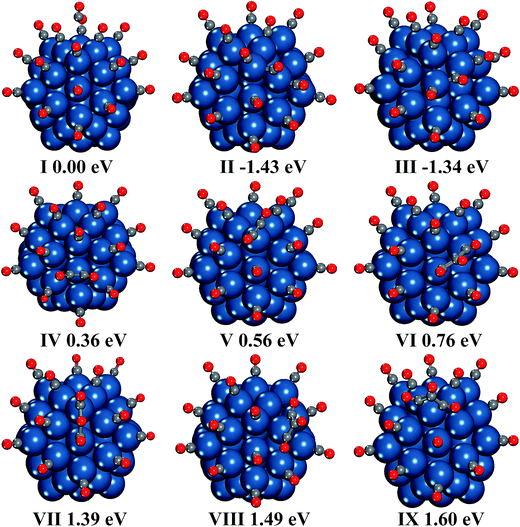
We also present other possible structures produced by two CO molecules in Fig. 1(V–VIII). It is found that two CO molecules can bond with each other by C–C bond to form CO dimer. C2O2 can be adsorbed on either two adjacent Pt atoms or one Pt atom, and the former case is preferred. In addition, the energy difference between C2O2 adsorption on two edge Pt atoms (structure VI in Fig. 1) and C2O2 adsorption on two adjacent corner and edge Pt atoms (structure V in Fig. 1) is very small (0.01 eV). However, the selectivity of adsorption site is relatively high in the cases of C2O2 adsorption on one Pt atom, because the energy of C2O2 adsorption on the corner site (structure VII in Fig. 1) is lower by 0.23 eV than that of C2O2 adsorption on the edge site (structure VIII in Fig. 1).
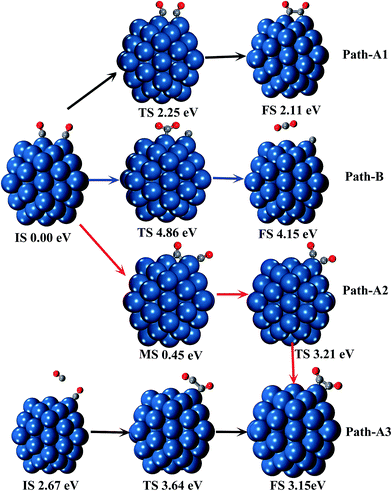
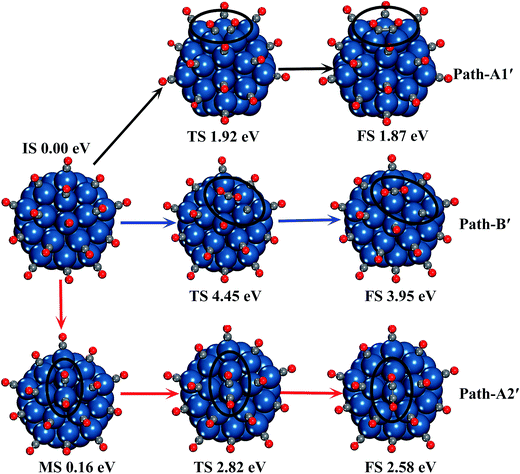
The reaction processes of C2O2 formation and CO disproportionation are investigated. The structure of two CO molecules adsorbed on two adjacent corner and edge Pt atoms is selected as the reactant of all the reactions between CO* adsorbates. Another reaction path of CO dimerization between one CO* and one free CO is also considered. In each reaction, we considered several structures of products to find out the most stable products. All the relative energies of structures involved in the reactions are depicted in Fig. 2, and the geometrical parameters of all the minima and transition states are presented in Fig. S2 in the ESI.† The disproportionation of CO on the Pt surface is found to be one-step reaction by overcoming a very high energy barrier (4.86 eV), which indicates CO2 is very unlikely to be generated by CO disproportionation on Pt surface. This result coincides with the conclusion of the experiments of temperature programmed desorption (TPD) of CO adsorption on the Pt surface.66,67 In the experiments, little CO2 produced by disproportionation of CO is detected.
The formation of C2O2 adsorbed on one Pt atom by two CO* undergoes two steps. First, the CO adsorbed on the edge Pt atom moves to the B1 site (seen in Fig. S1†). During this step, no transition state is observed, and the process is endothermic by 0.45 eV. Then the bridge CO overcomes an energy barrier of 2.76 eV to migrate to the corner site and form the C2O2. During the whole reaction process, the energy barrier is 3.21 eV. The CO adsorbed on the corner Pt atom can capture one free CO molecule to form C2O2 with overcoming a much lower energy barrier of 0.97 eV.
The formation of C2O2 adsorbed on two adjacent corner and edge Pt atoms takes place in only one step, and the energy barrier is 2.25 eV. The C–C distance is 1.60 Å in the product, which is 0.16 Å longer than that in the C2O2 adsorbed on one corner Pt atom. However, the Pt–C bonds are shorter by 0.01–0.02 Å in this case.
According to the potential energy surface of neutral C2O2 in the previous study, it costs at least 69 kcal mol−1 (2.99 eV) for two singlet state CO molecules to form isolated C2O2.29 As can be seen from above, when Pt55 cluster is involved in the formation of C2O2, the necessary energy is dramatically decreased.
 | ||
| Fig. 3 Geometrical structures of three CO molecules adsorption and trimerization on the Pt55 cluster. | ||
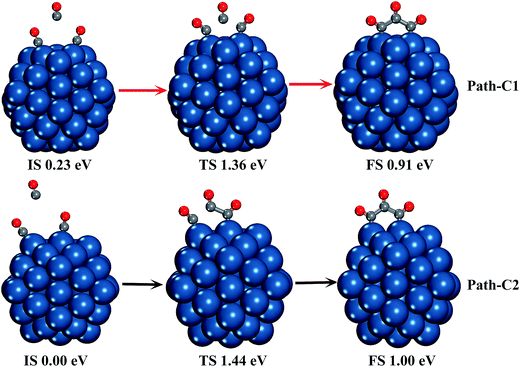
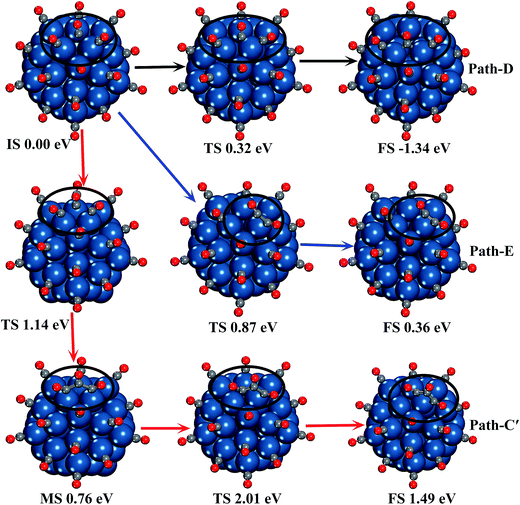
Moreover, in order to verify if C3O3 can be formed by two CO* molecules capturing a free CO molecule, we have searched the potential energy surface of the reaction process by the CI-NEB method. Because the preferred adsorption sites of two CO* molecules and C3O3 are different, we considered two paths of C3O3 formation on two Pt atoms, as presented in Fig. 4. The geometrical parameters of all the minima and transition states involved in the reaction paths are shown in Fig. S3.†
As can be seen in Fig. 4, both reactions are one-step processes, and the CO* adsorbed on two adjacent top edge Pt atoms (Path-C1) is better at capturing an additional CO to form C3O3 by overcoming a lower energy barrier of 1.13 eV. In Path-C1, the free CO bonds with the two adsorbed CO molecules at the same time. While in the other process, the free CO bonds with the CO molecules adsorbed on the edge site first, then it combines with the corner CO.
On the other hand, isolated C3O3 is also very unstable. Using HF, G2, and CBS methods, the symmetry of isolated C3O3 (cyclopropanetrione) is D3h with the three C atoms forming an equilateral triangle. The energy of the C3O3 triangular molecule is 323–383 kJ mol−1 (3.45–3.97 eV) higher than that of three CO molecules.68 However, no stationary point of C3O3 is found using DFT and other high level computational method in previous studies.33 The free C3O3 obtained in this study is a ring-opening compound, and no cyclopropanetrione adsorbed on the Pt55 cluster is found. Although C3O3 can also be adsorbed at one corner Pt atom, the C–C distance between two terminal C atoms is 2.33 Å, which is too large to form C–C bond. The energy increases by only 0.91 eV when one free CO molecule bonds with two CO* molecules.
Similarly, we still investigated the dimerization and disproportionation processes between the CO* at high CO coverage. Two different dimerization paths and one disproportionation path are performed. The reaction paths are depicted in Fig. 6, and the geometrical parameters of all the minima and transition states involved in the reaction paths are shown in Fig. S4.† The three reaction processes change significantly with high CO coverage effect, although the dimerization and disproportionation of CO on two Pt atoms remain to be one-step reaction and the dimerization of CO on one Pt atom is still a two-steps reaction. The active sites of CO dimerization and disproportionation on two atoms are changed into two adjacent edge Pt atoms. On the other hand, all the reactions become easier because of lower energy barriers: the energy barrier of CO disproportionation decreases from 4.86 eV to 4.45 eV, and for the CO dimerization on two edge Pt atoms it decreases from 2.25 eV to 1.92 eV. Besides, no transition state is observed in the first step of C2O2 formation on the corner Pt atom, and the energy barrier decreases from 3.21 eV to 2.82 eV in the whole reaction process.
 | ||
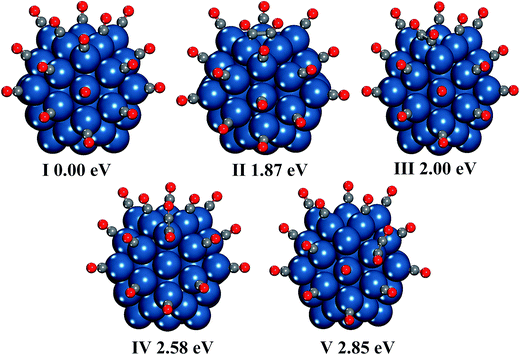
| Fig. 7 Geometrical structures of seventeen CO molecules adsorption on five facets of the Pt55 cluster. The energy of the structure (I) containing one free CO molecule is set at 0.00 eV. | ||
At the high CO coverage, it is found the free CO can also interact with CO* molecules to form the CO dimer and trimer, as shown in Fig. 7(IV–IX). For the dimerization between the free CO molecule and CO*, the C2O2 prefers to be adsorbed on the edge Pt atom rather than the corner Pt atom, which is contrary to the studies of C2O2 adsorption on one Pt atom in the Pt55(CO)2 and Pt55(CO)16. For the C3O3, the favorite adsorption site is still two edge Pt atoms. It should be emphasized that the C3O3 can also be adsorbed on one edge Pt atom, as shown in Fig. 7(VIII), which is not observed without the CO coverage effect. Meanwhile, the C3O3 prefers to be adsorbed on one edge Pt atom rather than one corner Pt atom.
The processes of the free CO molecule reacting with CO* to form CO polymers are illustrated in Fig. 8, and the adsorption process of the free CO molecule to the B2 site is also presented. The geometrical parameters of all the minima and transition states involved in the reaction paths are shown in Fig. S5.† It is observed that the free CO molecule can be easily adsorbed at the B2 site by overcoming an energy barrier of 0.32 eV (Path-E in Fig. 8), although each of the Pt atoms near the adsorption site has adsorbed one CO molecule. In terms of the calculated results of NEB, the CO molecule adsorbed at the B2 site cannot directly bond with two adjacent CO molecules to form the C3O3. In Path-C′, the free CO molecule migrates to two neighboring CO molecules adsorbed on two edge Pt atoms to form the C3O3. The energy barrier of the C3O3 formation is 1.14 eV, which is decreased by 0.22 eV compared with the C3O3 formation without the high CO coverage effect. Furthermore, the mobility of this newly formed C3O3 is also observed: the C3O3 adsorbed on two edge Pt atoms can be adsorbed on one edge Pt atom by overcoming an energy barrier of 1.25 eV, and the two C–C bonds are shortened to 1.56 Å. In the whole reaction process of forming the C3O3 adsorbed on one edge Pt atom, the energy barrier is 2.01 eV. The formation of C2O2 on one edge Pt atom is also found to be slightly easier with overcoming an energy barrier of 0.87 eV, which is the lowest energy barrier among all the considered reaction process of CO polymer formation.
3.2. Electronic properties of C2O2 adsorption on Pt55 cluster
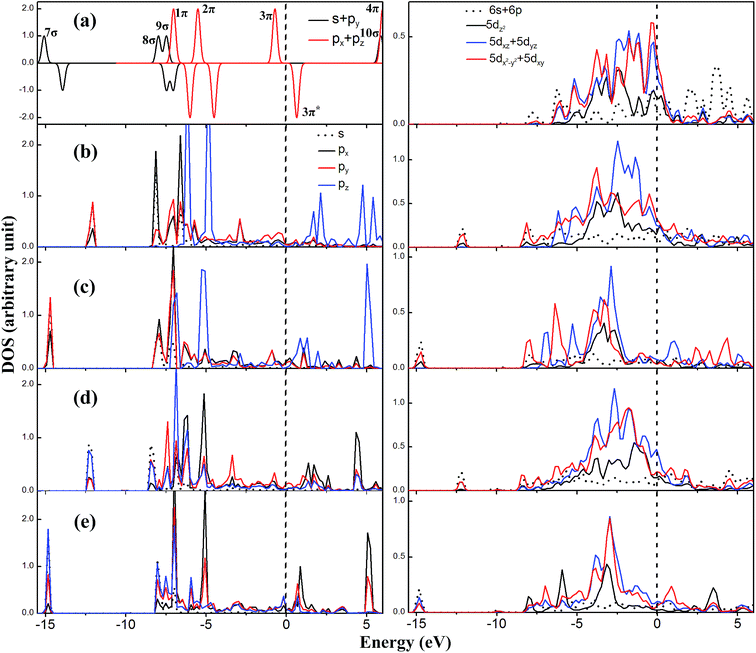
The electronic structure such as density of states (DOS) can present deep insight into the interaction between the adsorbate and metal surface. In this section we use the DOS to explain how the C2O2 is adsorbed on the Pt55 cluster with or without the CO coverage effect.In Fig. 9, we plot the projected density of states (PDOS) of the C2O2 and the Pt atoms before and after the C2O2 adsorption. The relevant molecular orbitals of one free C2O2 molecule are plotted in Fig. S6 in the ESI.† The two uppermost occupied orbitals of the free C2O2 are two singly-occupied 3π orbitals, represented as 3π(*). Because the ground state of the free C2O2 molecule is a triplet, we plot its DOS of spin up and spin down at the same time. While the DOS of spin up and spin down are the same not only for the adsorbed C2O2 but also for the Pt atoms after C2O2 adsorption, we used the spin polarized calculation method. Thus we plotted only the DOS of spin up for the adsorbed C2O2 and the bonding Pt atoms.
As can be obtained from Fig. 9(b), when the C2O2 is adsorbed on the two neighboring corner and edge Pt atoms, the two carbon atoms re-hybridize from sp to sp2. What is noteworthy is that only the 8σ orbital shifts to lower energy by about 0.3 eV compared with the free C2O2, but the other orbitals shift to higher energy. For example, the 7σ orbital shifts to higher energy by as much as 2.0 eV. The shift to higher energy arises from the elongated C–C bond length (1.61 Å), which weakens the interaction between the two C atoms. For the Pt atoms connected with the C2O2, their d orbitals interact with the 8σ orbital of C2O2, resulting in the shift to lower energy of these orbitals. The 8σ orbital dominates the interaction between the C2O2 and Pt atoms. On the other hand, in according to the newly formed peaks of the DOS of Pt at −12.0, −6.0, −5.0 and 2.0 eV, other orbitals of the C2O2, including 7σ, 9σ, 1π, 2π and 3π, are also found to participate in the interaction between the adsorbate and the metal surface. The molecular orbitals of the C2O2 after adsorption on two Pt atoms are exhibited in Fig. 10. As can be seen from the Fig. 10, the π orbitals in z-axis are broken after C2O2 adsorption. Since the 8σ and 9σ orbital are anti-bonding orbitals in the C2O2, the interactions between the two orbitals and the Pt cluster increase the stability of the C2O2 with donating the electron from the 8σ and 9σ orbitals to the metal. The interactions of the π orbitals of C2O2 with the Pt cluster decrease the stability of C2O2 because of the electron donation from the 1π and 2π orbitals to the empty metal orbitals and the electron back donation from the Pt cluster to the empty 3π* orbitals. However, the 8σ orbital dominates the electron transfer due to its strong interaction with the Pt cluster. Thus the C2O2 transfers 0.13 electrons to the Pt55 cluster in the light of the Mulliken population analysis.
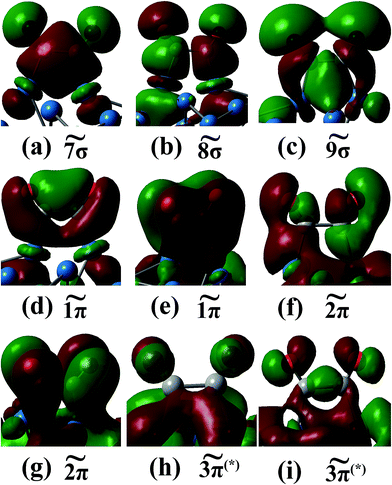 | ||
| Fig. 10 7σ (a), 8σ (b), 9σ (c), 1π (d and e), 2π (f and g) and 3π(*) (h and i) orbitals of C2O2 when C2O2 is adsorbed on two Pt atoms. | ||
In Fig. 9(c), when the C2O2 is adsorbed on one corner Pt atoms, the two carbon atoms also re-hybridize from sp to sp2. All the orbitals except the 8σ shift to lower energy compared with C2O2 adsorbed on two Pt atoms. There are two reasons leading to this fact. The first one is that the C–C bond length is 1.44 Å, which is much shorter, resulting in the enhancement of the interaction between the two carbon atoms. The second one is that the interactions between the orbitals of C2O2 and the metal orbitals also are strengthened, as can be seen from that all the bonding orbitals of the Pt atom dramatically shift to lower energy. But the orbital overlap between the 8σ and the metal orbitals is not increased, and the energy of 8σ is 0.3 eV higher. The bonding orbitals of the C2O2 adsorbed on one corner Pt atom are depicted in Fig. S7 in the ESI.† It is worth noting that the interaction between the empty 3π* of C2O2 and the d orbitals of Pt is relatively strong due to part of 3π* state dramatically shifting to lower energy near the Fermi level, leading to more electrons transferred from Pt atom to empty 3π* orbital.
When the CO coverage effect is taken into account, all the bonding interactions between the C2O2 and Pt are weakened, because the intensities of the peaks below −5.0 eV are weakened especially for the Pt, as shown in the Fig. 9(d) and (e). The adsorption of CO can change the geometrical and electronic properties of the Pt cluster. The repulsive interaction between adsorbed CO elongates the bond length of neighboring Pt atoms, and every CO molecule donates as many as 0.3 electrons to the Pt cluster. The high CO coverage weakens the adsorption of C2O2 on the Pt55 cluster, and the adsorption of CO is also weakened as found in previous studies.61,65
4. Conclusions
In summary, the adsorption properties of CO, C2O2 and C3O3 on the icosahedral Pt55 cluster were investigated. It is found the CO prefers to be adsorbed at the corner site without the effect of CO coverage, while the high CO coverage leads to the CO migration from the top site to the bridge site. The CO can dimerize to form a C–C bond not only on two Pt atoms but also on one Pt atom. In the former case, the C–C bond length is 1.60 Å, and in the latter case it is 1.44 Å. The dimer prefers to be adsorbed on two Pt atoms rather than one Pt atom. The preference can be ascribed to the stronger interaction between the 8σ orbital of C2O2 and the 5d orbitals of Pt for the C2O2 adsorbed on two Pt atoms, and this interaction increases the stability of C2O2. But for the C2O2 adsorbed on one Pt atom, the interaction between the 3π* orbital of the C2O2 and the 5d orbitals of Pt is stronger, resulting in the decrease of the C2O2 stability. The C3O3 also prefers to be adsorbed on two Pt atoms rather than one Pt atom. The high CO coverage can weaken the adsorption strength of the CO adsorption, which is beneficial for the CO polymerization, although the adsorption strength of CO polymers is also weakened. When the CO coverage is increased to 1 ML, the energy barrier of the CO dimerization by two CO* is decreased from 2.26 eV to 1.92 eV, which is still much too high. However, the CO* can capture one free CO molecule to form the C2O2 and C3O3 by overcoming much lower energy barriers. At the high CO coverage, the energy barrier of the CO dimerization by one CO* and one free CO molecule is decreased from 0.97 eV to 0.87 eV, and the energy barrier of the CO trimerization by two one CO* and one free CO molecule is decreased from 1.36 eV to 1.14 eV. Thus, the CO polymerization between CO* and free CO should be considered especially when the CO coverage or CO pressure is high. In this study, we have investigated the static properties of the CO dimerization and trimerization on the Pt cluster. In order to gain more accurate results about the stabilities of CO dimer and trimer, pressure and temperature should be considered, and that is what we will do in our next study.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 21376013).References
- F. Fischer and H. Tropsch, Brennst.-Chem., 1926, 7, 97–116 CAS.
- H. Pichler and H. Schulz, Chem. Ing. Tech., 1970, 42, 1162–1174 CrossRef CAS.
- X. Dai and C. Yu, J. Nat. Gas Chem., 2008, 17, 365–368 CrossRef CAS.
- M. Ojeda, R. Nabar, A. U. Nilekar, A. Ishikawa, M. Mavrikakis and E. Iglesia, J. Catal., 2010, 272, 287–297 CrossRef CAS.
- F. M. Hoffmann and J. L. Robbins, J. Electron Spectrosc. Relat. Phenom., 1987, 45, 421–428 CrossRef CAS.
- C. F. Huo, J. Ren, Y. W. Li, J. Wang and H. Jiao, J. Catal., 2007, 249, 174–184 CrossRef CAS.
- O. R. Inderwildi, S. J. Jenkins and D. A. King, J. Phys. Chem. C, 2008, 112, 1305–1307 CAS.
- G. A. Morgan Jr, D. C. Sorescu, T. Zubkov and J. T. Yates Jr, J. Phys. Chem. B, 2004, 108, 3614–3624 CrossRef.
- O. R. Inderwildi, S. J. Jenkins and D. A. King, J. Am. Chem. Soc., 2007, 129, 1751–1759 CrossRef CAS PubMed.
- O. R. Inderwildi, S. J. Jenkins and D. A. King, Angew. Chem., Int. Ed., 2008, 47, 5253–5255 CrossRef CAS PubMed.
- T. Zubkov, G. A. Morgan Jr, J. T. Yates Jr, O. Kühlert, M. Lisowski, R. Schillinger, D. Fick and H. J. Jänsch, Surf. Sci., 2003, 526, 57–71 CrossRef CAS.
- C. Y. Fan, H. P. Bonzel and K. Jacobi, J. Chem. Phys., 2003, 118, 9773–9782 CrossRef CAS.
- Q. Ge and M. Neurock, J. Phys. Chem. B, 2006, 110, 15368–15380 CrossRef CAS PubMed.
- M. M. Kappes and R. H. Staley, J. Am. Chem. Soc., 1981, 103, 1286–1287 CrossRef CAS.
- M. Araki and V. Ponec, J. Catal., 1976, 44, 439–448 CrossRef CAS.
- C. S. Kellner and A. T. Bell, J. Catal., 1981, 67, 175–185 CrossRef CAS.
- D. S. Santilli and D. G. Castner, Energy Fuels, 1989, 3, 8–15 CrossRef CAS.
- V. V. Ordomsky, B. Legras, K. Cheng, S. Paul and A. Y. Khodakov, Catal. Sci. Technol., 2015, 5, 1433–1437 CAS.
- K. J. P. Schouten, Y. Kwon, C. J. M. van der Ham, Z. Qin and M. T. M. Koper, Chem. Sci., 2011, 2, 1902–1909 RSC.
- J. H. Montoya, A. A. Peterson and J. K. Nørskov, ChemCatChem, 2013, 5, 737–742 CrossRef CAS.
- J. H. Montoya, C. Shi, K. Chan and J. K. Nørskov, J. Phys. Chem. Lett., 2015, 6, 2032–2037 CrossRef CAS PubMed.
- F. Calle-Vallejo and M. T. M. Koper, Angew. Chem., 2013, 125, 7423–7426 CrossRef.
- M. Gattrell, N. Gupta and A. Co, J. Electroanal. Chem., 2006, 594, 1–19 CrossRef CAS.
- K. J. P. Schouten, E. P. Gallent and M. T. M. Koper, ACS Catal., 2013, 3, 1292–1295 CrossRef CAS.
- K. J. P. Schouten, Z. Qin, E. P. Gallent and M. T. M. Koper, J. Am. Chem. Soc., 2012, 134, 9864–9867 CrossRef CAS PubMed.
- F. Jia, X. Yu and L. Zhang, J. Power Sources, 2014, 252, 85–89 CrossRef CAS.
- S. Shironita, K. Karasuda, K. Sato and M. Umeda, J. Power Sources, 2013, 240, 404–410 CrossRef CAS.
- T. Hatsukade, K. P. Kuhl, E. R. Cave, D. N. Abram and T. F. Jaramillo, Phys. Chem. Chem. Phys., 2014, 16, 13814–13819 RSC.
- D. Schröder, C. Heinemann, H. Schwarz, J. N. Harvey, S. Dua, S. J. Blanksby and J. H. Bowie, Chem.–Eur. J., 1998, 4, 2550–2557 CrossRef.
- G. P. Raine, H. F. Schaefer III and R. C. Haddon, J. Am. Chem. Soc., 1983, 105, 194–198 CrossRef CAS.
- T. Watanabe, Y. Ishida, T. Matsuo and H. Kawaguchi, J. Am. Chem. Soc., 2009, 131, 3474–3475 CrossRef CAS PubMed.
- R. B. Wyrwas and C. C. Jarrold, J. Am. Chem. Soc., 2006, 128, 13688–13689 CrossRef CAS PubMed.
- X. Bao, X. Zhou, C. F. Lovitt, A. Venkatraman, D. A. Hrovat, R. Gleiter, R. Hoffmann and W. T. Borden, J. Am. Chem. Soc., 2012, 134, 10259–10270 CrossRef CAS PubMed.
- H. Jiao and H. S. Wu, J. Org. Chem., 2003, 68, 1475–1479 CrossRef CAS PubMed.
- S. Schlemmer, A. Luca, J. Glosik and D. Gerlich, J. Chem. Phys., 2002, 116, 4508–4516 CrossRef CAS.
- K. Hiraoka, T. Mori and S. Yamabe, J. Chem. Phys., 1991, 94, 2697–2703 CrossRef CAS.
- R. M. Morris and K. J. Klabunde, J. Am. Chem. Soc., 1983, 105, 2633–2639 CrossRef CAS.
- S. M. Mansell, N. Kaltsoyannis and P. L. Arnold, J. Am. Chem. Soc., 2011, 133, 9036–9051 CrossRef CAS PubMed.
- O. P. Lam and K. Meyer, Angew. Chem., Int. Ed., 2011, 50, 9542–9544 CrossRef CAS PubMed.
- O. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green and N. Hazari, Science, 2006, 311, 829–831 CrossRef CAS PubMed.
- O. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green and N. Hazari, J. Am. Chem. Soc., 2006, 128, 9602–9603 CrossRef CAS PubMed.
- K. P. Kuhl, E. R. Cave, D. N. Abram and T. F. Jaramillo, Energy Environ. Sci., 2012, 5, 7050–7059 CAS.
- S. S. Sung and R. Hoffmann, J. Am. Chem. Soc., 1985, 107, 578–584 CrossRef CAS.
- B. Hammer and J. K. Nørskov, Adv. Catal., 2000, 45, 71–129 CAS.
- Z. Zhu, G. Melaet, S. Axnanda, S. Alayoglu, Z. Liu, M. Salmeron and G. A. Somorjai, J. Am. Chem. Soc., 2013, 135, 12560–12563 CrossRef CAS PubMed.
- C. K. Tsung, J. N. Kuhn, W. Huang, C. Aliaga, L. I. Hung, G. A. Somorjai and P. Yang, J. Am. Chem. Soc., 2009, 131, 5816–5822 CrossRef CAS PubMed.
- H. Song, R. M. Rioux, J. D. Hoefelmeyer, R. Komor, K. Niesz, M. Grass, P. Yang and G. A. Somorjai, J. Am. Chem. Soc., 2006, 128, 3027–3037 CrossRef CAS PubMed.
- J. H. Ryu, S. S. Han, D. H. Kim, G. Henkelman and H. M. Lee, ACS Nano, 2011, 5, 8515–8522 CrossRef CAS PubMed.
- E. Aprà and A. Fortunelli, J. Phys. Chem. A, 2003, 107, 2934–2942 CrossRef.
- X. Zhao, R. Zhang, L. Ling and B. Wang, Appl. Surf. Sci., 2014, 320, 681–688 CrossRef CAS.
- S. Dobrin, Phys. Chem. Chem. Phys., 2012, 14, 12122–12129 RSC.
- J. VandeVondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing and J. Hutter, Comput. Phys. Commun., 2005, 167, 103–128 CrossRef CAS.
- G. Lippert, J. Hutter and M. Parrinello, Mol. Phys., 1997, 92, 477–488 CrossRef CAS.
- J. P. Perdew, K. Burke and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 16533–16539 CrossRef CAS.
- J. VandeVondele and J. Hutter, J. Chem. Phys., 2007, 127, 114105 CrossRef PubMed.
- S. Goedecker, M. Teter and J. Hutter, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 1703–1710 CrossRef CAS.
- B. G. Pfrommer, M. Côté, S. G. Louie and M. L. Cohen, J. Comput. Phys., 1997, 131, 233–240 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jonsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- P. J. Feibelman, B. Hammer, J. K. Nørskov, F. Wagner, M. Scheffler, R. Stumpf, R. Watwe and J. Dumesic, J. Phys. Chem. B, 2001, 105, 4018–4025 CrossRef CAS.
- P. Gruene, A. Fielicke, G. Meijer and D. M. Rayner, Phys. Chem. Chem. Phys., 2008, 10, 6144–6149 RSC.
- M. Gajdoš, A. Eichler and J. Hafner, J. Phys.: Condens. Matter, 2004, 16, 1141–1164 CrossRef.
- R. Brako and D. Šokčević, Surf. Sci., 1998, 401, L388–L394 CrossRef CAS.
- F. Tao, S. Dag, L. W. Wang, Z. Liu, D. R. Butcher, M. Salmeron and G. A. Somorjai, Nano Lett., 2009, 9, 2167–2171 CrossRef CAS PubMed.
- F. Tao, S. Dag, L. W. Wang, Z. Liu, D. R. Butcher, H. Bluhm, M. Salmeron and G. A. Somorjai, Science, 2010, 327, 850–853 CrossRef CAS PubMed.
- B. T. Loveless, C. Buda, M. Neurock and E. Iglesia, J. Am. Chem. Soc., 2013, 135, 6107–6121 CrossRef CAS PubMed.
- K. Foger and J. R. Anderson, Appl. Surf. Sci., 1979, 2, 335–351 CrossRef CAS.
- T. Yamamoto, T. Shido, S. Inagaki, Y. Fukushima and M. Ichikawa, J. Phys. Chem. B, 1998, 102, 3866–3875 CrossRef CAS.
- G. Corkran and D. W. Ball, J. Mol. Struct.: THEOCHEM, 2004, 668, 171–178 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25989d |
| This journal is © The Royal Society of Chemistry 2016 |