Hydrophobic chain modified low molecular weight polyethylenimine for efficient antigen delivery†
Hui Wanga,
Jian Chen*a,
Jiajun Yinga,
Yuhong Xua and
Ruilong Sheng*bc
aSchool of Pharmacy, Shanghai Jiao Tong University, Dongchuan Road 800, Shanghai, 200240, China. E-mail: chenjian@sjtu.edu.cn
bKey Laboratory of Synthetic and Self-assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, CAS. Lingling Road 345, Shanghai, 200032, China. E-mail: rlsheng@mail.sioc.ac.cn
cDepartment of Chemistry, Université de Montréal, Succursale Centre-ville, Montréal, Quebéc H3C3J7, Canada
First published on 26th January 2016
Abstract
The development of new therapeutic vaccines for the efficient induction of cellular immunity by antigen cross-presentation in antigen-presenting cells, especially dendritic cells, is regarded as a promising approach to prime antigen-specific T cell responses and to destroy tumor cells. We synthesized low molecular weight polyethylenimine (C12-PEI) with a modified hydrophobic lipid chain by the direct coupling of 1-bromododecane with low molecular weight polyethylenimine (MW 423 Da) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. This was bound with model antigen ovalbumin (OVA) to form sphere-like polyplex nanoparticles (size 158–302 nm; zeta potential −18.4 to −13.1 mV) by electrostatic interaction at an C12-PEI/OVA weight ratio of 0.03–0.08. The in vitro cytotoxicity of the C12-PEI/OVA nanoparticles was strongly dependent on the weight ratio of the C12-PEI and OVA. The antigen cross-presentation effect (IL-2 secretion) of the C12-PEI/OVA polyplexes was also dependent on the weight ratio. Optimized cross-presentation was achieved on the C12-PEI/OVA-0.07/1 polyplexes, which was 5.3-fold higher than that of the free OVA solution and 1.9-fold higher than that of the PEI/OVA polyplexes. The intracellular distribution of the C12-PEI/rhodamine-labeled OVA polyplexes demonstrated the lysosome localization effect. These results indicated that the synthesized C12-PEI cationic polymer with a modified hydrophobic lipid chain could potentially be used as a therapeutic vaccine carrier for delivery of the OVA antigen.
1 molar ratio. This was bound with model antigen ovalbumin (OVA) to form sphere-like polyplex nanoparticles (size 158–302 nm; zeta potential −18.4 to −13.1 mV) by electrostatic interaction at an C12-PEI/OVA weight ratio of 0.03–0.08. The in vitro cytotoxicity of the C12-PEI/OVA nanoparticles was strongly dependent on the weight ratio of the C12-PEI and OVA. The antigen cross-presentation effect (IL-2 secretion) of the C12-PEI/OVA polyplexes was also dependent on the weight ratio. Optimized cross-presentation was achieved on the C12-PEI/OVA-0.07/1 polyplexes, which was 5.3-fold higher than that of the free OVA solution and 1.9-fold higher than that of the PEI/OVA polyplexes. The intracellular distribution of the C12-PEI/rhodamine-labeled OVA polyplexes demonstrated the lysosome localization effect. These results indicated that the synthesized C12-PEI cationic polymer with a modified hydrophobic lipid chain could potentially be used as a therapeutic vaccine carrier for delivery of the OVA antigen.
1. Introduction
Cancer immunotherapy, in which therapeutic vaccines are used to enhance the power and specificity of the immune system to target tumors, is considered to be a promising tool in cancer treatment.1–4 The molecular identification of human cancer antigens as therapeutic vaccines has allowed the development of antigen-specific immunotherapy; in this vaccination approach, the provision of an antigen together with an adjuvant to prime therapeutic antigen-specific T cells in vivo5 is essential. However, the antigens used as cancer vaccines have a protein-based architecture and are easily degraded by proteases or absorbed by serum proteins in the blood circulation system. Antigen proteins could also induce a predominantly antibody response rather than an antigen-specific T lymphocyte response. These side-effects greatly decrease their immuno-therapeutic efficacy and limit their clinical applications against tumors.6,7 Thus the key issues in the stimulation of cellular immunity against tumor cells is developing new carrier materials:8 (1) to protect the protein-based antigens/vaccines against rapid biodegradation or absorption in the bloodstream; and (2) to control the antigen processing of the vaccines inside antigen-presenting cells (APCs) through the major histocompatibility complex (MHC) I pathway.The development of new synthetic polymeric materials for use as vaccine carriers has attracted increasing attention in cancer immunotherapy.9,10 Positively charged cationic polymers/lipids have been used as polymeric carriers to absorb or load negatively charged therapeutic antigen/vaccine proteins11,12 via electrostatic interactions. Polyethylenimines (PEIs) are a well-known series of commercially available, low cost and highly efficient polymeric gene vectors with a high positive charge density and unique “proton sponge” effect,13,14 which facilitates disruption of the endosome/lysosome compartments and the release of DNA into the cytosol. This feature also makes them potential candidates to bind or load negatively charged antigen proteins and to promote endosome/lysosome escape for the efficient therapeutic delivery of antigens or vaccines. We previously reported the in vitro delivery of ovalbumin (OVA) antigen using commercially available PEI-25k as the polymeric vaccine carrier. We found that the PEI-25k/antigen polyplex nanoparticles improved the antigen cross-presentation efficiency in professional APCs.15 However, the design and synthesis of new PEI-based polymeric antigen/vaccine carriers that can form nano-scale antigen carriers or antigen complexes with good stability in solution and enhanced antigen cross-presentation efficiency in APCs are still challenging. There is a need to further elucidate the correlation between the polymer structures and their capability to deliver antigens and vaccines. Many modification approaches have been developed in the field of gene delivery to enhance the utility of PEI-based gene carriers/vectors. Hydrophobic modifications of lower molecular weight PEI have been shown to be promising approaches to achieve a high cell membrane penetration capability and highly efficient gene delivery. Bhattacharya and co-workers16 found that hydrophobic cholesterol-modified low molecular weight PEI enhanced the cellular uptake capability of the pDNA-loaded polyplexes and improved the efficiency of gene delivery. This could be explained by the hydrophobic interaction between the hydrophobic aliphatic chains and the lipophilic cell membrane, which resulted in enhanced cellular uptake of the polyplexes. There have been several successful studies of hydrophobic modifications to PEI polymers in gene delivery systems, demonstrating that modification of the hydrophobic lipid chains combined with low molecular weight PEI could improve the efficiency of gene delivery.17,18 Thus it is expected that the development of new PEI-based therapeutic vaccine delivery systems by the hydrophobic modification of low molecular weight PEI might also give rise to a higher cell membrane penetration capability and higher efficiency of vaccine delivery.
Based on this hypothesis, we designed and synthesized a hydrophobic-modified low molecular weight PEI (C12-PEI) by direct coupling of 1-bromododecane with low molecular weight PEI (MW 423 Da) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The C12-PEI polymer was used as a vaccine carrier to deliver the model antigen OVA into dendritic cells (DCs) and to further prime the immune response of therapeutic antigen-specific T cells by antigen cross-presentation (Scheme 1). The average particle size, zeta potential, morphology and stability in solution of the C12-PEI/OVA complexes were measured by dynamic light scattering (DLS), transmission electron microscopy (TEM) and atomic force microscopy (AFM). The cytotoxicity of the C12-PEI/OVA polyplexes was evaluated by the CCK-8 assay. DCs derived from bone marrow and Kb-restricted OVA-specific T cells (RF33.70) were used as efficient antigen-presenting model cell lines to evaluate the cross-presentation efficiency using an IL-2 secretion assay. The intracellular localization of the C12-PEI/rhodamine-labeled OVA polyplexes was recorded by confocal fluorescent microscopy.
1 molar ratio. The C12-PEI polymer was used as a vaccine carrier to deliver the model antigen OVA into dendritic cells (DCs) and to further prime the immune response of therapeutic antigen-specific T cells by antigen cross-presentation (Scheme 1). The average particle size, zeta potential, morphology and stability in solution of the C12-PEI/OVA complexes were measured by dynamic light scattering (DLS), transmission electron microscopy (TEM) and atomic force microscopy (AFM). The cytotoxicity of the C12-PEI/OVA polyplexes was evaluated by the CCK-8 assay. DCs derived from bone marrow and Kb-restricted OVA-specific T cells (RF33.70) were used as efficient antigen-presenting model cell lines to evaluate the cross-presentation efficiency using an IL-2 secretion assay. The intracellular localization of the C12-PEI/rhodamine-labeled OVA polyplexes was recorded by confocal fluorescent microscopy.
2. Materials and methods
2.1. Materials
Low molecular weight polyethylenimine (PEI, branched, MW 423 Da), PEI-25k (branched, MW 25 kDa) and antigen OVA were purchased from Sigma-Aldrich (St Louis, MO, USA). 1-Bromododecane (99.0%) was purchased from Aladdin-Reagent (Shanghai, China). Mouse granulocyte-macrophage colony-stimulating factor and mouse interleukin (IL-4) were purchased from R&D Systems (Minneapolis, MN, USA). Rhodamine red succinimidyl ester was purchased from Life Technology (Shanghai, China). Cell culture media were obtained from Gibco Invitrogen (Carlsbad, CA, USA). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Shanghai, China).Kb-restricted OVA-specific hybridoma T cells RF33.70 (specific for the peptide of OVA257–264) were kindly provided by Professor Xuetao Cao from the Second Military Medical University (Shanghai, China). These cells were maintained in RPMI-1640 culture medium with 10% heat-inactivated fetal bovine serum and antibiotics. Male C57BL/6 mice (H-2b) (5–6 weeks old) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China) and were housed in a specific pathogen-free room at the School of Pharmacy, Shanghai Jiaotong University. All experiments were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University School of Pharmacy (Shanghai, China). DCs derived from bone marrow were prepared using a previously reported procedure.19 Bone marrow from C57BL/6 mice was collected and cultured in RPMI-1640 complete medium (10% heat-inactivated fetal bovine serum, 100 U mL−1 penicillin, 100 U mL−1 streptomycin) supplemented with 10 ng mL−1 mouse granulocyte-macrophage colony-stimulating factor and 1 ng mL−1 mouse IL-4. Non-adherent cells were removed after 3 days and the adherent cells were replanted in fresh RPMI-1640 complete medium supplemented with mouse granulocyte-macrophage colony-stimulating factor and IL-4. The non-adherent and loosely adherent cells were harvested as DCs after another 3 days.
2.2. Methods
C12-PEI. 1H NMR (CDCl3, δ ppm): 4.31–4.12 (br, NH, PEI), 3.17–2.18 (m, 34H, NHCH2CH2NH, PEI), 1.41–1.06 (m, 40H, CH2, alkyl chain), 0.94–0.72 (t, 6H, CH3, alkyl chain). FTIR (cm−1): 3255, 2920, 2850, 2814, 1639, 1456, 1353, 1302, 1157, 1119, 1008, 938, 770, 720.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.08
1 and 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, and then incubating at room temperature for 20 min. For the TEM measurements, the as-prepared polyplex solution was dropped onto a carbon-coated copper grid and left to dry in air at room temperature for 24 h. The nanoparticle morphologies were then recorded using a transmission electron microscope (JEM-1230, JEOL, Japan) operated at an accelerating voltage of 80 kV. For the AFM measurements, the as-prepared C12-PEI/OVA-0.07/1 polyplexes were diluted 10× with ultrapure water and then deposited onto freshly cleaved mica. After evaporating the water to give a dry sample, the morphology of the C12-PEI/OVA-0.07/1 nanoparticles was observed by AFM (Nanonavi E-Sweep, Seiko Instruments).
1, respectively, and then incubating at room temperature for 20 min. For the TEM measurements, the as-prepared polyplex solution was dropped onto a carbon-coated copper grid and left to dry in air at room temperature for 24 h. The nanoparticle morphologies were then recorded using a transmission electron microscope (JEM-1230, JEOL, Japan) operated at an accelerating voltage of 80 kV. For the AFM measurements, the as-prepared C12-PEI/OVA-0.07/1 polyplexes were diluted 10× with ultrapure water and then deposited onto freshly cleaved mica. After evaporating the water to give a dry sample, the morphology of the C12-PEI/OVA-0.07/1 nanoparticles was observed by AFM (Nanonavi E-Sweep, Seiko Instruments).3. Results and discussion
3.1. Synthesis of the C12-PEI cationic lipopolymer
It is known that the introduction of a hydrophobic block/moiety to cationic amphiphiles may facilitate the formation of nano-aggregates, improve the stability of the gene/drug payloads in solution, and enhance cell membrane fusion and permeation.20,21 Cao and Feng22 found that doxorubicin conjugated with lipid containing vitamin E had a lower IC50 value than free doxorubicin. In an effort to improve the performance of gene vectors, we previously found that the presence of a hydrophobic poly(L-lactic acid) block in some amphiphilic triblock dendritic poly(L-lysine)-b-poly(L-lactic acid)-b-dendritic poly(L-lysine) blocks could significantly enhance the DNA binding affinity and efficiency of transfection.23 In the work reported here, to mimic the membrane fusion lipids bearing double alkyl chains (such as DOPE and DOTMA), we synthesized a low molecular weight PEI (C12-PEI) bearing a double alkyl chain by direct coupling of 1-bromododecane with low molecular weight PEI (MW 423 Da) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.16 The reaction mixture was heated under comparatively mild conditions (70 °C) for 24 h until the 1-bromododecane reacted completely (monitored by 1H NMR); the final product (C12-PEI) was obtained as yellowish oil without further purification. The synthetic routes for the C12-PEI cationic polymers from the 423 Da PEI and 1-bromododecane are shown in Scheme 1; the structural characterization of the C12-PEI cationic polymer by 1H NMR and FTIR is described in detail in the experimental section (see Fig. S1†). In the 1H NMR spectrum, the proton signals at δ 0.94–0.72 ppm (–CH3) and δ 1.41–1.06 ppm (–CH2–) represent the alkyl chains and the signal at δ 3.17–2.18 was identified as the –CH2–CH2– protons of the 423 Da PEI. Strong absorption bands at 2920, 2850 and 2814 cm−1 (νCH3 and νCH2) and a comparably broad absorption band at 3500–3150 cm−1 (νNH2 and νNH) were seen in the IR spectrum. These results confirmed the successful preparation of the C12-PEI lipopolymer.
1 molar ratio.16 The reaction mixture was heated under comparatively mild conditions (70 °C) for 24 h until the 1-bromododecane reacted completely (monitored by 1H NMR); the final product (C12-PEI) was obtained as yellowish oil without further purification. The synthetic routes for the C12-PEI cationic polymers from the 423 Da PEI and 1-bromododecane are shown in Scheme 1; the structural characterization of the C12-PEI cationic polymer by 1H NMR and FTIR is described in detail in the experimental section (see Fig. S1†). In the 1H NMR spectrum, the proton signals at δ 0.94–0.72 ppm (–CH3) and δ 1.41–1.06 ppm (–CH2–) represent the alkyl chains and the signal at δ 3.17–2.18 was identified as the –CH2–CH2– protons of the 423 Da PEI. Strong absorption bands at 2920, 2850 and 2814 cm−1 (νCH3 and νCH2) and a comparably broad absorption band at 3500–3150 cm−1 (νNH2 and νNH) were seen in the IR spectrum. These results confirmed the successful preparation of the C12-PEI lipopolymer.
3.2. Average particle size and zeta potential of the C12-PEI/OVA polyplexes
It is known that the average particle size and the zeta potential of the drug/gene payloads influence their cytotoxicity, the delivery efficiency of the drug/gene and the intracellular uptake and trafficking behavior.24,25 The average particle size and zeta potential of the C12-PEI/OVA polyplexes in solution were determined by DLS. The C12-PEI/OVA polyplexes were prepared by mixing OVA (1 mg mL−1) with various amounts of C12-PEI (0.03–0.08 mg mL−1) in HEPES buffer (10 mM, pH 7.4). Fig. 1A shows the dependence of the average particle sizes of the C12-PEI/OVA polyplexes in aqueous solution on the weight ratio. The C12-PEI cationic polymer could compact OVA into relatively small nanoparticles (diameter 158–302 nm) with a narrow polydispersity index (PDI 0.1–0.28) in the weight ratio range of 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.08
1 to 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (see Fig. S2† for the DLS curve at 0.07
1 (see Fig. S2† for the DLS curve at 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It was difficult for the C12-PEI and OVA to form complexes when the weight ratio was >0.08
1). It was difficult for the C12-PEI and OVA to form complexes when the weight ratio was >0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At C12-PEI/OVA weight ratios of 0.03
1. At C12-PEI/OVA weight ratios of 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.08
1 to 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the zeta potential of the C12-PEI/OVA particles was in the range −18.4 to −13.1 mV (Fig. 1B). The negative charge of the complexes showed that these nanoparticles were different from other PEI-based nano-delivery systems.15 It is suggested that, in aqueous solution, the C12-PEI polymer first assembled into micelles with a hydrophobic core containing an alkyl chain and a hydrophilic, positively charged shell containing 423 Da PEI. The negatively charged OVA proteins could be absorbed (loaded) onto the micelle surface to form the negatively charged C12-PEI/OVA nanoparticles.
1, the zeta potential of the C12-PEI/OVA particles was in the range −18.4 to −13.1 mV (Fig. 1B). The negative charge of the complexes showed that these nanoparticles were different from other PEI-based nano-delivery systems.15 It is suggested that, in aqueous solution, the C12-PEI polymer first assembled into micelles with a hydrophobic core containing an alkyl chain and a hydrophilic, positively charged shell containing 423 Da PEI. The negatively charged OVA proteins could be absorbed (loaded) onto the micelle surface to form the negatively charged C12-PEI/OVA nanoparticles.
 | ||
| Fig. 1 (A) Average particle size and polydispersity index (PDI) and (B) zeta potential of the C12-PEI/OVA complexes. | ||
3.3. Morphology of the C12-PEI/OVA polyplexes
To further elucidate the assembly/aggregation features of the as-prepared C12-PEI/OVA polyplexes, the morphology of the C12-PEI/OVA-0.07/1 and C12-PEI/OVA-0.08/1 polyplexes was observed by TEM and AFM. The C12-PEI/OVA-0.07/1 and C12-PEI/OVA-0.08/1 polyplexes were prepared by mixing the OVA solution (1 mg mL−1) with the C12-PEI polymer solution at C12-PEI/OVA weight ratios of 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.08
1 and 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, and were then incubated at 37 °C for 20 min. From the TEM images in Fig. 2, it can be seen that the C12-PEI/OVA-0.07/1 and C12-PEI/OVA-0.08/1 polyplexes were uniformly dispersed sphere-like nanoparticles with average particle sizes of around 40–70 nm; a similar sphere-like morphology and particle size (60–100 nm) were also observed by AFM (Fig. S3†). Larger particle sizes (∼290 nm) were measured by DLS than observed by TEM/AFM, which could be a result of shrinkage of the nanoparticles during drying of the C12-PEI/OVA polyplexes before observation by TEM/AFM.26
1, respectively, and were then incubated at 37 °C for 20 min. From the TEM images in Fig. 2, it can be seen that the C12-PEI/OVA-0.07/1 and C12-PEI/OVA-0.08/1 polyplexes were uniformly dispersed sphere-like nanoparticles with average particle sizes of around 40–70 nm; a similar sphere-like morphology and particle size (60–100 nm) were also observed by AFM (Fig. S3†). Larger particle sizes (∼290 nm) were measured by DLS than observed by TEM/AFM, which could be a result of shrinkage of the nanoparticles during drying of the C12-PEI/OVA polyplexes before observation by TEM/AFM.26
 | ||
| Fig. 2 Transmission electron microscopy (TEM) images of the C12-PEI/OVA polyplex nanoparticles: (A–C) C12-PEI/OVA-0.07/1 at different magnifications; and (D) C12-PEI/OVA-0.08/1. | ||
3.4. Stability of the C12-PEI/OVA polyplexes in solution
The stability of the antigen/vaccine payload in solution is an essential parameter for both the in vitro and in vivo delivery of therapeutic substances.27,28 We evaluated the stability of the C12-PEI/OVA polyplexes by DLS under various conditions. Fig. 3 shows that the average particle size of the C12-PEI/OVA-0.07/1 polyplexes was still in the range 298–312 nm in the RPMI-1640 culture medium at 37 °C, indicating that the binding of C12-PEI with OVA could form stable C12-PEI/OVA-0.07/1 polyplexes. This binding could be a result of the induced self-aggregation of the hydrophobic dodecyl chain, which could subsequently lead to the formation of micelles with hydrophobic lipid cores.29 This would decrease the dissociation of the C12-PEI/OVA-0.07/1 polyplexes in the RPMI-1640 culture solution. We have previously found that the stability of nanoparticles in a series of triblock polymer (DLn-PLLAm-DLn)/DNA polyplexes could be enhanced by lengthening the hydrophobic PLLA block.23 We further examined the stability of the C12-PEI/OVA polyplexes by DLS in 0.9% NaCl and 10 mM HEPES (Fig. S4†). The size of the polyplexes could be maintained at around 250–420 nm within 2 days. These results showed that the C12-PEI/OVA polyplexes could maintain their average particle size at a comparatively stable level under various solution conditions for up to 2 days. This short-term stability at room temperature might make them suitable for potential use in a vaccine formula in practical applications. | ||
| Fig. 3 Change with time of the average particle size and PDI of the C12-PEI/OVA-0.07/1 polyplexes during incubation at 37 °C in RPMI-1640 culture medium. | ||
3.5. In vitro cytotoxicity of the C12-PEI/OVA polyplexes by CCK-8 assay
The development of carriers with low cytotoxicity is considered to an essential prerequisite for safe therapeutic applications. The in vitro cytotoxicity of the C12-PEI/OVA polyplexes was evaluated in DCs by a CCK-8 assay30 and the polyplexes formed by 25 kDa PEI and OVA (PEI/OVA) was used as the control. Fig. 4 shows that PEI/OVA had a high cytotoxicity (about 54% survival) at a weight ratio of 0.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas C12-PEI/OVA had a very low cytotoxicity (about 99% survival) at a weight ratio of 0.04
1, whereas C12-PEI/OVA had a very low cytotoxicity (about 99% survival) at a weight ratio of 0.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.05
1 to 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which might be due to the lower molecular weight of the cationic PEI decreasing the disruption of the cell membrane.31 However, as the mass ratio increased to 0.06
1, which might be due to the lower molecular weight of the cationic PEI decreasing the disruption of the cell membrane.31 However, as the mass ratio increased to 0.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and higher, the cytotoxicity significantly increased. As the C12-PEI/OVA polyplexes were negatively charged nanoparticles, the cytotoxicity could be attributed to their intracellular metabolism instead of the disruption of the cell membrane induced by a cationic charge.32 The results also suggested that the in vitro cytotoxicity largely depended on the weight ratio of C12-PEI/OVA in the polyplex nanoparticles.
1 and higher, the cytotoxicity significantly increased. As the C12-PEI/OVA polyplexes were negatively charged nanoparticles, the cytotoxicity could be attributed to their intracellular metabolism instead of the disruption of the cell membrane induced by a cationic charge.32 The results also suggested that the in vitro cytotoxicity largely depended on the weight ratio of C12-PEI/OVA in the polyplex nanoparticles.
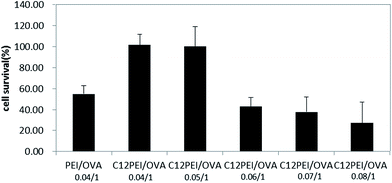 | ||
| Fig. 4 In vitro cytotoxicity of the C12-PEI/OVA polyplexes in DCs by the CCK-8 assay; PEI/OVA-0.04/1 was used as a control. | ||
3.6. Antigen cross-presenting efficiency by the C12-PEI/OVA polyplexes
The antigen cross-presenting efficiency of the C12-PEI/OVA polyplex nanoparticles was further evaluated with the co-culture of antigen-pulsed DCs (pre-incubated with the C12-PEI/OVA polyplexes) with RF33.70 hybridoma cells specific for the MHCI/OVA257–264 complex on the cell surface.33 Once the antigens from the DCs had been cross-presented by the MHC I complexes, they could interact with specifically with these T cells and up-regulate the secretion of IL-2. We have previously reported the delivery of OVA antigen using PEI-25k as the polymer carrier, which gave rise to enhanced IL-2 secretion and improved the antigen cross-presentation efficiency of the DCs.15 Here, we evaluated the secretion of IL-2 induced by C12-PEI/OVA; the polyplex formed by PEI-25k and OVA (PEI/OVA) was used as a reference. Fig. 5 shows that the secretion of IL-2 by RF33.70 cells in the C12-PEI/OVA polyplex nanoparticles at weight ratios of 0.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.09
1 to 0.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was about 1.2–5.3-fold higher than that of the free OVA solution. The highest secretion of IL-2 from the RF33.70 cells was observed when they were incubated with the C12-PEI/OVA polyplex nanoparticles at a weight ratio of 0.07; the secretion of IL-2 was 1.9-fold higher than that seen with the PEI/OVA-0.04/1 polyplex nanoparticles. These results suggest that the weight ratio of the C12-PEI/OVA polyplex nanoparticles was a critical factor in antigen presentation and the comparatively high IL-2 secretion indicated that the C12-PEI/OVA polyplex might be a promising tool for the delivery of OVA antigen.
1 was about 1.2–5.3-fold higher than that of the free OVA solution. The highest secretion of IL-2 from the RF33.70 cells was observed when they were incubated with the C12-PEI/OVA polyplex nanoparticles at a weight ratio of 0.07; the secretion of IL-2 was 1.9-fold higher than that seen with the PEI/OVA-0.04/1 polyplex nanoparticles. These results suggest that the weight ratio of the C12-PEI/OVA polyplex nanoparticles was a critical factor in antigen presentation and the comparatively high IL-2 secretion indicated that the C12-PEI/OVA polyplex might be a promising tool for the delivery of OVA antigen.
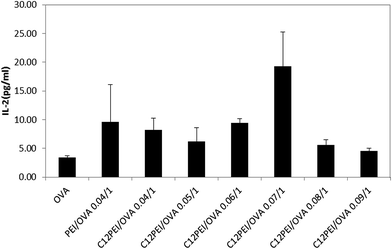 | ||
| Fig. 5 Secretion of IL-2 by RF33.70 cells after incubation with different amounts of the C12-PEI/OVA polyplexes to stimulate the DCs. | ||
3.7. Intracellular distribution of the C12-PEI/rhodamine-labeled OVA polyplexes
To further understand the intracellular distribution of the C12-PEI/OVA polyplexes in DCs, rhodamine-labeled OVA (Rho-OVA) was prepared and mixed with C12-PEI to prepare the C12-PEI/Rho-OVA polyplexes, which were then incubated with DCs for 30 min and then observed by fluorescence microscopy. Fig. 6 shows the fluorescence images (400×) obtained after the incubation of C12-PEI/Rho-OVA polyplexes for 30 min with DCs. The red fluorescence of the rhodamine dye showed the successful and efficient cellular internalization of the C12-PEI/Rho-OVA polyplexes. Fluorescent images for the polyplexes after incubation periods of 15 min, 30 min and 1 h were recorded (Fig. S5†) and showed that the C12-PEI/Rho-OVA polyplexes could be taken up into the DC cells within a relatively short time of 30 min. Most of the red fluorescent Rho-OVA co-localized with the green fluorescent dye Lysotracker Green, indicating that a lysosome localization effect34 was involved in the cellular trafficking process for the C12-PEI/Rho-OVA polyplexes. This may lead to efficient intracellular OVA release by the well-known “proton sponge” effect of low molecular weight 423 Da PEI. The rapid uptake might be caused by the relatively small nanoparticle size and the membrane fusion capability of the OVA-loaded nanoparticles. It has been reported that some cationic lipids bearing alkyl chains are inclined to be taken up via a pathway mediated by lipid rafts or caveolae.35 We therefore suggest that the C12-PEI/Rho-OVA polyplexes might be internalized through a lipid raft or caveolae dependent pathway to finally give rise to efficient delivery of the OVA antigen. Further investigation of the intracellular trafficking mechanisms of the antigen/polymer carrier polyplexes has been carried out in our laboratory. | ||
| Fig. 6 Fluorescence images of the intracellular distribution of the C12-PEI/Rho-OVA polyplexes in DCs after incubation for 30 min. | ||
4. Conclusion
We have designed and synthesized a hydrophobic-modified low molecular weight PEI (C12-PEI) by direct coupling of 1-bromododecane with low molecular weight PEI (MW 423 Da) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The synthesized C12-PEI could bind or load OVA antigen at weight ratios of 0.03
1 molar ratio. The synthesized C12-PEI could bind or load OVA antigen at weight ratios of 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.08
1 to 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form C12-PEI/OVA polyplexes with an average particle size range of 158–302 nm and a zeta potential range of −18.4 to −13.1 mV. Stability studies showed that the C12-PEI/OVA-0.07/1 polyplexes had short-term storage stability (up to 2 days) in RPMI-1640 culture medium, 0.9% NaCl and 10 mM HEPES at room temperature. The CCK-8 assay results indicated that the cytotoxicity of the C12-PEI/OVA polyplexes greatly depended on the weight ratio of C12-PEI/OVA and the antigen cross-presentation (IL-2 secretion) effect of the C12-PEI/OVA polyplexes was also dependent on the weight ratio. Optimized IL-2 secretion was achieved on the C12-PEI/OVA-0.07/1 polyplexes and was 5.3-fold higher than with free OVA and 1.9-fold higher than on the PEI/OVA-0.04/1 polyplexes. The intracellular distribution of the C12-PEI/Rho-OVA polyplexes showed the obvious lysosome localization effect of the polyplexes, suggesting that the “lysosomal escape” effect of the C12-PEI/OVA polyplexes may play an essential role in subsequent OVA antigen release and cross-presentation.
1 to form C12-PEI/OVA polyplexes with an average particle size range of 158–302 nm and a zeta potential range of −18.4 to −13.1 mV. Stability studies showed that the C12-PEI/OVA-0.07/1 polyplexes had short-term storage stability (up to 2 days) in RPMI-1640 culture medium, 0.9% NaCl and 10 mM HEPES at room temperature. The CCK-8 assay results indicated that the cytotoxicity of the C12-PEI/OVA polyplexes greatly depended on the weight ratio of C12-PEI/OVA and the antigen cross-presentation (IL-2 secretion) effect of the C12-PEI/OVA polyplexes was also dependent on the weight ratio. Optimized IL-2 secretion was achieved on the C12-PEI/OVA-0.07/1 polyplexes and was 5.3-fold higher than with free OVA and 1.9-fold higher than on the PEI/OVA-0.04/1 polyplexes. The intracellular distribution of the C12-PEI/Rho-OVA polyplexes showed the obvious lysosome localization effect of the polyplexes, suggesting that the “lysosomal escape” effect of the C12-PEI/OVA polyplexes may play an essential role in subsequent OVA antigen release and cross-presentation.
This study has demonstrated that the synthesized hydrophobic C12-PEI lipopolymer with modified with an alkyl chain could potentially be used as a therapeutic vaccine carrier for the delivery of the OVA antigen. The hydrophobic chain number and length, the degree of unsaturation of the hydrophobic chain, the charge density, and the linkers and linkage bonds may play important roles in determining the cytotoxicity and delivery efficiency of drugs, genes or vaccines.36 The correlation between the chemical structural factors of the nanocarriers37 and the vaccine delivery properties are under further investigation in our laboratory.
Acknowledgements
The authors are grateful for partial funding from the National Science Foundation of China (NSFC 81001406 and 21372251) and the Funds for Scientific Research by Minhang Committee of Science and Technology (No. 2015MH138). We also acknowledge the generous help from the employees of Instrumental Analysis Centre of Shanghai Jiao Tong University. Dr Ruilong Sheng thanks the Chinese Academy of Sciences (CAS) for the CAS–Canada Young Scientist Visiting Scholarship and Youth Innovation Promotion Association for sponsorship.References
- K. Palucka and J. Banchereau, Nat. Rev. Cancer, 2012, 12, 265–277 CrossRef CAS PubMed.
- K. Palucka and J. Banchereau, Immunity, 2013, 39, 38–48 CrossRef CAS PubMed.
- K. Palucka, H. Ueno and J. Banchereau, J. Immunol., 2011, 186, 1325–1331 CrossRef CAS PubMed.
- K. Palucka, H. Ueno, J. Fay and J. Banchereau, J. Intern. Med., 2011, 269, 64–73 CrossRef CAS PubMed.
- J. Schlom, J. Natl. Cancer Inst., 2012, 104, 599–613 CrossRef CAS PubMed.
- S. Beg, A. Samad, I. Nazish, R. Sultana, M. Rahman, M. Z. Ahmad and M. Akbar, Curr. Drug Targets, 2013, 14, 123–137 CrossRef CAS PubMed.
- A. Bolhassani, S. Safaiyan and S. Rafati, Mol. Cancer, 2011, 10, 1–20 CrossRef PubMed.
- T. Suksamran, T. Ngawhirunpat, T. Rojanarata, W. Sajomsang, T. Pitaksuteepong and P. Opanasopit, Int. J. Pharm., 2013, 448, 19–27 CrossRef CAS PubMed.
- J. Leleux and K. Roy, Adv. Healthcare Mater., 2013, 2, 72–94 CrossRef CAS PubMed.
- P. Sahdev, L. J. Ochyl and J. J. Moon, Pharm. Res., 2014, 31, 2563–2582 CrossRef CAS PubMed.
- S. Foster, C. L. Duvall, E. F. Crownover, A. S. Hoffman and P. S. Stayton, Bioconjugate Chem., 2010, 21, 2205–2212 CrossRef CAS PubMed.
- Y. Yoshizaki, E. Yuba, N. Sakaguchi, K. Koiwai, A. Harada and K. Kono, Biomaterials, 2014, 35, 8186–8196 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualch, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297–7301 CrossRef CAS.
- A. Zintchenko, A. Philipp, A. Dehshahri and E. Wagner, Bioconjugate Chem., 2008, 19, 1448–1455 CrossRef CAS PubMed.
- J. Chen, Z. Li, H. Huang, Y. Yang, Q. Ding, J. Mai, W. Guo and Y. Xu, Int. J. Nanomed., 2011, 6, 77–84 CrossRef CAS PubMed.
- A. Bajaj, P. Kondaiah and S. Bhattacharya, Bioconjugate Chem., 2008, 19, 1640–1651 CrossRef CAS PubMed.
- P. Y. Teo, C. Yang, J. L. Hedrick, A. C. Engler, D. J. Coady, S. Ghaem-Maghami, A. J. T. George and Y. Y. Yang, Biomaterials, 2013, 34, 7971–7979 CrossRef CAS PubMed.
- G. Guo, L. Zhou, Z. Chen, W. Chi, X. Yang, W. Wang and B. Zhang, Int. J. Pharm., 2013, 450, 44–52 CrossRef CAS PubMed.
- J. Pan, M. Zhang, J. Wang, Q. Wang, D. Xia, W. Sun, L. Zhang, H. Yu, Y. Liu and X. Cao, Immunol. Lett., 2004, 94, 141–151 CrossRef CAS PubMed.
- B. Wang, C. He, C. Tang and C. Yin, Biomaterials, 2011, 32, 4630–4638 CrossRef CAS PubMed.
- H. Akita, R. Ishiba, R. Togashi, K. Tange, Y. Nakai, H. Hatakeyama and H. Harashima, J. Controlled Release, 2015, 200, 97–105 CrossRef CAS PubMed.
- N. Cao and S. S. Feng, Biomaterials, 2008, 29, 3856–3865 CrossRef CAS PubMed.
- Y. Li, L. Cui, Q. Li, L. Jia, Y. Xu, Q. Fang and A. Cao, Biomacromolecules, 2007, 8, 1409–1416 CrossRef CAS PubMed.
- S. Honary and F. Zahir, Trop. J. Pharm. Res., 2013, 12, 255–264 Search PubMed.
- J. C. Sunshine, D. Y. Peng and J. J. Green, Mol. Pharm., 2012, 9, 3375–3383 CrossRef CAS PubMed.
- R. Sheng, K. Xia, J. Chen, Y. Xu and A. Cao, J. Biomater. Sci., Polym. Ed., 2013, 24, 1935–1951 CrossRef CAS PubMed.
- A. K. Jain, A. K. Goyal, P. N. Gupta, K. Khatri, N. Mishra, A. Mehta, S. Mangal and S. P. Vyas, J. Controlled Release, 2009, 136, 161–169 CrossRef CAS PubMed.
- P. E. Makidon, S. S. Nigavekar, A. U. Bielinska, N. Mank, A. M. Shetty, J. Suman, J. Knowlton, A. Myc, T. Rook and J. R. Baker Jr, J. Aerosol Med. Pulm. Drug Delivery, 2010, 23, 77–89 CrossRef CAS PubMed.
- R. Sheng, T. Luo, Y. Zhu, H. Li, J. Sun, S. Chen, W. Sun and A. Cao, Biomaterials, 2011, 32, 3507–3519 CrossRef CAS PubMed.
- J. E. Weldon, L. Xiang, O. Chertov, I. Margulies, R. J. Kreitman, D. J. FitzGerald and I. Pastan, Blood, 2009, 113, 3792–3800 CrossRef CAS PubMed.
- R. Sheng, T. Luo, H. Li, J. Sun, Z. Wang and A. Cao, Bioorg. Med. Chem., 2013, 21, 6366–6377 CrossRef CAS PubMed.
- C. Srinivasan and D. J. Burgess, J. Controlled Release, 2009, 136, 62–70 CrossRef CAS PubMed.
- S. Van Meirvenne, L. Straetman, C. Heirman, M. Dullaers, C. De Greef, V. Van Tendeloo and K. Thielemans, Cancer Gene Ther., 2002, 9, 787–797 CrossRef CAS PubMed.
- R. Sheng, T. Luo, H. Li, J. Sun, Z. Wang and A. Cao, Colloids Surf., B, 2014, 116, 32–40 CrossRef CAS PubMed.
- Y. U. Bae, B. K. Kim, J. W. Park, Y. B. Seu and K. O. Doh, Mol. Pharm., 2012, 9, 3579–3585 CrossRef CAS PubMed.
- R. Sheng, F. An, Z. Wang, M. Li and A. Cao, RSC Adv., 2015, 5, 12338–12345 RSC.
- M. Zhu, G. Nie, H. Meng, T. Xia, A. Nel and Y. Zhao, Acc. Chem. Res., 2013, 46, 622–631 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25919c |
| This journal is © The Royal Society of Chemistry 2016 |

