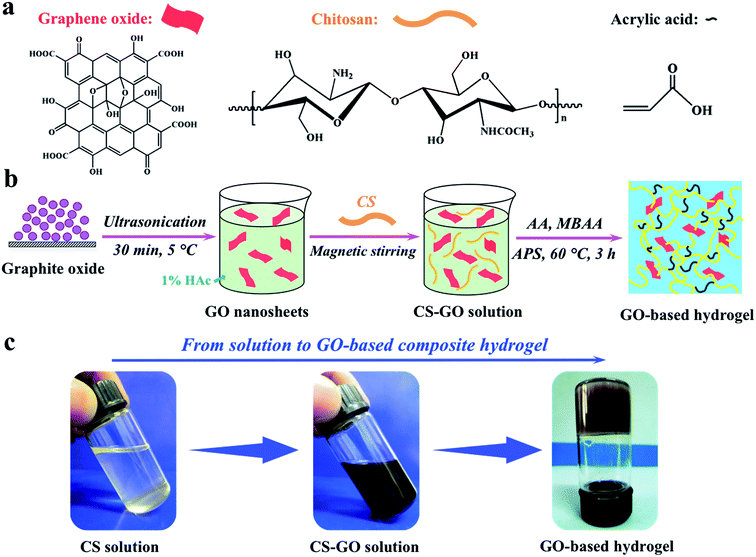
Graphene oxide-based composite hydrogels with self-assembled macroporous structures†
Yiwan Huang‡
a,
Ming Zeng*abc,
Zijian Fenga,
Die Yina,
Qingyu Xua and
Liren Fanab
aFaculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, P. R. China. E-mail: mingzeng@cug.edu.cn; zengming318@163.com; Tel: +86 18064129618
bEngineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan 430074, P. R. China
cZhejiang Research Institute, China University of Geosciences, Wuhan 430074, P. R. China
First published on 18th December 2015
Abstract
The self-assembly technique provides a new and simple route for designing porous hydrogels. At present, most of the studies in graphene oxide (GO)–polymer hydrogels are concentrated on mechanical reinforcement. Developing a self-assembled GO-based porous hydrogel along with swelling and mechanical merits is still challenging, yet very interesting and desirable for practical applications. Herein, we report self-assembled GO-based macroporous composite hydrogels by integrating GO sheets and chitosan-based hydrogel networks. GO sheets, containing adequate hydrophilic functional groups, can be dispersed well and thereby they form self-assembled supramolecular structures with polymer chains by effective intermolecular interactions (e.g., hydrogen bonding, electrostatic attraction or covalent bonding). Surprisingly, an extremely low amount (0.05–0.30 wt%) of GO can remarkably affect the architecture of hydrogel networks, leading to the formation of macroporous composite hydrogels. On the whole, the GO-based polymer composite hydrogels possess both macroporous structures (10–100 μm) and enhanced mechanical performance, yet can still retain the similar swelling properties of their parent polymeric hydrogel. Therefore, this study provides a simple route for fabricating porous hydrogels, which could find some potential applications in wastewater treatment or biomedical engineering.
Introduction
Polymeric hydrogels are water-rich, cross-linked hydrophilic polymer networks.1 They have attracted great interest all over the world in recent years, because of their promising potential in diverse application fields, such as biological systems, medical engineering, food industry, agriculture, and environmental treatment.2–6 To realize the practical applications in some fields, for example in a biomedical engineering system or wastewater treatment, gel scientists usually prefer to design hydrogels with porous structures for effectively accepting cells or adsorbing heavy metals as well as organic dyes.1,6 Several strategies have been extensively utilized to fabricate porous hydrogels, including freeze-drying,7 foaming techniques,8 and phase separation.9It is worth noting that the self-assembly technique provides a new and simple route for designing porous gels. Self-assembly has been generally recognized as one of the most amazing strategies for integrating materials with unique structures or some new functions, mainly on the basis of noncovalent intermolecular interactions.10–12 Recently, this self-assembly strategy has also been utilized to develop hydrogels or aerogels with unique porous structures from a good kind of nanoscale building block, namely graphene and its derivative graphene oxide (GO).13–16 In fact, GO is also a unique candidate for fabricating self-assembled composite hydrogels because GO contains adequate hydrophilic groups (i.e., –OH, –C–O–C–, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH groups) on the surface and at the edge.17 It is easy for GO to form an effective interfacial interaction with a hydrophilic matrix through noncovalent or covalent bonds. According to the unprecedented physical and chemical properties (e.g., high surface area, high thermal conductivity, mechanical strength, and excellent electrical conductivity) of GO sheets, most work in GO–polymer hydrogels mainly concentrates on mechanical enhancement or realizing some functions.6,18–23 Interestingly, a self-assembled GO/poly(vinyl alcohol) hydrogel has also been prepared and has shown good pH-sensitivity, where GO acts as a 2D molecular building block.24 Furthermore, self-assembled hydrogels from GO and DNA biomolecules have been fabricated with in situ formed porous structures, exhibiting a high dye-adsorption capacity.25 However, the current work on self-assembled macroporous GO–polymer hydrogels is still limited.
O and –COOH groups) on the surface and at the edge.17 It is easy for GO to form an effective interfacial interaction with a hydrophilic matrix through noncovalent or covalent bonds. According to the unprecedented physical and chemical properties (e.g., high surface area, high thermal conductivity, mechanical strength, and excellent electrical conductivity) of GO sheets, most work in GO–polymer hydrogels mainly concentrates on mechanical enhancement or realizing some functions.6,18–23 Interestingly, a self-assembled GO/poly(vinyl alcohol) hydrogel has also been prepared and has shown good pH-sensitivity, where GO acts as a 2D molecular building block.24 Furthermore, self-assembled hydrogels from GO and DNA biomolecules have been fabricated with in situ formed porous structures, exhibiting a high dye-adsorption capacity.25 However, the current work on self-assembled macroporous GO–polymer hydrogels is still limited.
Chitosan (CS), a cationic natural polysaccharide, bears abundant hydrophilic active groups (i.e., –OH and –NH2 groups), which can provide effective interaction sites with GO sheets, including hydrogen bonding, electrostatic attraction, or covalent bonding, for self-assembly.6,26–30 Yan et al. developed a supramolecular hydrogel by the self-assembly of chitosan chains with GO sheets; yet, its mechanical strength and swelling property were not satisfactory.31 Therefore, the development of novel self-assembled porous GO-based polymeric hydrogels combined with good mechanical as well as swelling properties is considered to be a very interesting and desirable study for their use in practical applications. It is noted that chitosan-grafted polymer hydrogels occupying cross-linking networks, e.g., chitosan-graft-poly(acrylic acid) (CS-g-PAA) hydrogel, have the advantages of satisfactory swelling and mechanical properties.32 Thus, chitosan-grafted polymer hydrogel is probably a good polymer matrix candidate for designing a novel self-assembled GO-based porous hydrogel with swelling and mechanical merits.26,32
In this work, we report self-assembled GO-based macroporous composite hydrogels by integrating GO sheets and CS-g-PAA polymeric networks, along with enhanced mechanical performance. In detail, the microstructure and its formation mechanism and the intermolecular interactions of the composite hydrogels are thoroughly discussed. Their swelling capacity, salt- and pH-sensitivities, as well as mechanical performance are also evaluated. This work therefore not only provides a simple path for fabricating porous hydrogels but also gives some insight into the formation of self-assembled polymer composite hydrogels via effective intermolecular interactions. The self-assembled macroporous composite hydrogels combined with mechanical and swelling merits could find some potential applications in wastewater treatment or in biomedical engineering.
Experimental
Materials
Natural graphite flake (23 μm, 99.99% purity) was purchased from Qingdao Guyu Graphite Co., Ltd. Chitosan (CS) with a molecular weight of 900 kDa and a deacetylation degree of 85% was obtained from Sigma-Aldrich. Acrylic acid (AA) was distilled under reduced pressure prior to use. Acetic acid (HAc), sodium hydroxide (NaOH), ammonium persulfate (APS), and N,N′-methylenebisacrylamide (MBAA) were of analytical grade and used as received. All the solutions in the experiments were prepared with deionized water.Preparation of GO sheets and GO/CS-g-PAA composite hydrogels
Graphite oxide was synthesized via a modified Hummers method described in our previous work.18,33 Briefly, to generate fully exfoliated GO sheets, 0.20 g of graphite oxide powder was dispersed into 100 mL of deionized water, followed by an ultrasonic treatment for 30 min to form a stable GO solution (yellow-brown color, 2 mg mL−1).For preparing GO/CS-g-PAA composite hydrogels, a desired amount of GO solution was added into 60 mL of 1% (v/v) HAc solution, followed by dissolving 1.00 g of CS powder in this solution at 20 °C. The viscosity of the mixed solution increased sharply and the solution changed into gel immediately. The gel solution was stirred overnight, and then treated by ultrasonication in an ice-water bath for 30 min to guarantee a homogenous dispersion. Afterward, 2 mL of 0.10 g mL−1 APS solution was added into the above solution with stirring at 60 °C. Ten minutes later, 7.20 g of AA and 0.20 g of MBAA were respectively added into the solution with stirring and then the reaction cell was kept at 60 °C for 2 h to complete the polymerization. The resultant products were allowed to cool down to ambient temperature and were then cut into small pieces, followed by the neutralization with aqueous NaOH solution to pH 8. Then, the products were washed with deionized water until the pH was 7 and were dehydrated with ethanol for 24 h. After complete dehydration, the hard products were vacuum-dried at 50 °C to a constant weight for characterization. By changing the weight ratio (0.05–0.80 wt%) of GO to AA, a series of GO/CS-g-PAA composite hydrogels were fabricated. The pure CS-g-PAA hydrogel was also obtained according to the above procedure, only without adding GO.
Swelling properties of GO/CS-g-PAA composite hydrogels
The swelling ratios of dried samples in deionized water, normal saline, salt solutions, and various pH (1–13) solutions were measured by a gravimetric method previously described.18,34 The different pH values of the solutions were adjusted by using HCl and NaOH solutions, where the ionic strength was kept constant (0.1 M) by adding a desired amount of NaCl. The swelling ratios (W) of hydrogel samples were calculated by W = (Ws − Wd)/Wd, where Ws was the weight of swollen samples and Wd was the weight of dried samples. W was calculated as grams of water per gram of samples. At least three measurements were carried out for each sample and the average value was calculated.Characterizations
Fourier transform infrared (FTIR) spectra of the dried samples were recorded on a Thermal Scientific Nicolet 6700 spectrometer using the KBr disk method in a wavenumber range of 4000–400 cm−1. Wide-angle X-ray diffraction (WXRD) measurements were performed on a Philips X'Pert Pro MPD X-ray diffractometer using Cu Kα radiation (λ = 15.405) in a diffraction angle range of 3–40°. The morphologies of the wet hydrogel samples were observed directly by using a Leica DM2500 P optical microscope (OM). The cross-section morphologies of dried hydrogel samples were obtained by using an FEI inspect F field emission scanning electron microscope (FESEM). Thermo-gravimetric analysis (TGA) and corresponding differential thermo-gravimetric analysis (DTG) of dried samples were conducted in a nitrogen atmosphere with a heating rate of 10 °C min−1 by using a Netzsch TG 209F1 instrument in a temperature range of 25–700 °C.Results and discussion
Formation of GO/CS-g-PAA composite hydrogels
To obtain homogeneous GO–polymer composite hydrogels, it is very important to not only guarantee a good dispersion of GO sheets in the pre-gel solution but also to keep this dispersion stable until the hydrogel is formed.35 As presented in Fig. 1a, the chemical structures of graphene oxide, chitosan, and acrylic acid are sketched out, respectively. The GO surface consists of sufficient hydrophilic functional groups (i.e., –COOH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, –C–O–C–, and –C–OH groups), which is considered to be very beneficial for its stable dispersion in water and also for self-assembly by effective intermolecular interactions with hydrophilic chitosan chains. Herein, the preparation procedure of composite hydrogels is also briefly described in Fig. 1b. It is expected that chitosan molecules should well dissolve in the aqueous GO solution and then form effective interactions with GO sheets. Indeed, as we observed, when adding CS powder into the GO solution, the viscosity of this mixed solution increased sharply and the solution changed into a gel immediately, directly suggesting the formation of a good CS–GO combination via effective intermolecular interaction, e.g., hydrogen bonding or electrostatic attraction. In fact, these strong interactions have already been confirmed as the driving force behind self-assembly to obtain the CS/GO nanocomposite films from CS and GO in aqueous media.27,29 The simultaneous improvement of the strength and toughness of the nanocomposite films could be attributed to the homogeneous dispersion and unidirectional alignment of GO sheets in the chitosan matrix, and the strong interfacial adhesion between GO and chitosan.27 After magnetic stirring, as shown in Fig. 1c, the above CS–GO gels became a viscous CS–GO solution again, and the GO sheets could be well dispersed in the chitosan solution. Then, the homogeneous GO/CS-g-PAA composite hydrogels could be formed subsequently after the graft polymerization of acrylic acid.
C–, –C–O–C–, and –C–OH groups), which is considered to be very beneficial for its stable dispersion in water and also for self-assembly by effective intermolecular interactions with hydrophilic chitosan chains. Herein, the preparation procedure of composite hydrogels is also briefly described in Fig. 1b. It is expected that chitosan molecules should well dissolve in the aqueous GO solution and then form effective interactions with GO sheets. Indeed, as we observed, when adding CS powder into the GO solution, the viscosity of this mixed solution increased sharply and the solution changed into a gel immediately, directly suggesting the formation of a good CS–GO combination via effective intermolecular interaction, e.g., hydrogen bonding or electrostatic attraction. In fact, these strong interactions have already been confirmed as the driving force behind self-assembly to obtain the CS/GO nanocomposite films from CS and GO in aqueous media.27,29 The simultaneous improvement of the strength and toughness of the nanocomposite films could be attributed to the homogeneous dispersion and unidirectional alignment of GO sheets in the chitosan matrix, and the strong interfacial adhesion between GO and chitosan.27 After magnetic stirring, as shown in Fig. 1c, the above CS–GO gels became a viscous CS–GO solution again, and the GO sheets could be well dispersed in the chitosan solution. Then, the homogeneous GO/CS-g-PAA composite hydrogels could be formed subsequently after the graft polymerization of acrylic acid.
Based on the above experimental observations, the self-assembled CS–GO combination formed prior to graft polymerization. Although the combined units are destroyed to some extent under magnetic stirring, it is supposed that the remaining CS–GO units probably affect the structure formation of composite hydrogels. Furthermore, the noncovalent intermolecular interactions between GO and the CS-g-PAA network in the composite hydrogels should still occur based on the chemical structure characteristics of the components. The above assumption prompts us to explore the microstructure and its formation mechanism of composite hydrogels in the following discussion.
Microstructure and formation mechanism of GO/CS-g-PAA composite hydrogels
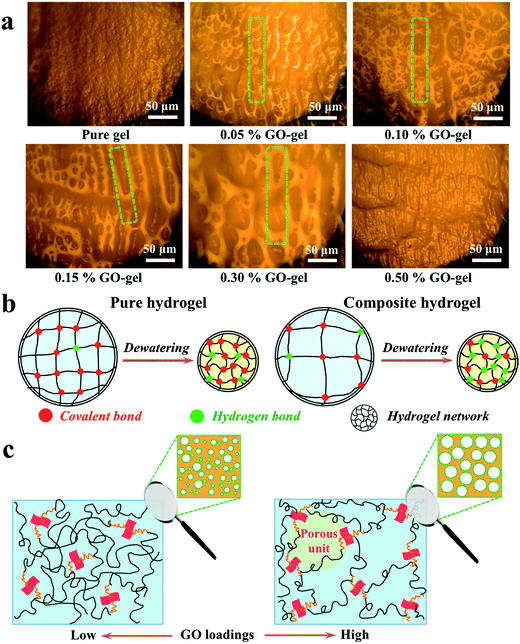
To study the microstructure and formation mechanism of GO/CS-g-PAA composite hydrogels, the morphologies of wet hydrogel samples were directly observed with an optical microscope, as shown in Fig. 2. It is interestingly found that the incorporation of GO into the CS-g-PAA pure hydrogel networks is beneficial for producing porous structures. The pure hydrogel exhibits a relatively dense gel network microstructure. As the amount of GO increases from 0.05 wt% to 0.30 wt%, however, the GO/CS-g-PAA composite hydrogels show interconnected macroporous structures, and the range of pore sizes is approximately 10–100 μm. Furthermore, with increasing the amount of GO, the pore size increases gradually and then parallel-aligned interconnected pores appear. These porous structures are very similar to the previous observation in the macroporous polyisobutylene gels.36 This result reveals that the effective interactions between GO sheets and the polymer chains do significantly affect the structure construction of the hydrogel matrix.As the amount of GO increases further (>0.30 wt%), however, the composite hydrogels become non-porous again, which might be attributed to both the increased cross-linking density of the hydrogel networks and the apparent aggregations of excessive GO sheets in the matrix. Therefore, a proper amount of GO addition can result in the formation of self-assembled macroporous structures in the composite hydrogel networks.
To briefly understand the intermolecular interactions between GO sheets and the hydrogel matrix, the cross-sections of vacuum-dried hydrogel samples were observed by FESEM, and the morphologies with different magnifications are presented in Fig. 3. It was found that the microstructures of hydrogel samples after dewatering were quite different from that observed with the optical microscope. In the FESEM observation, the CS-g-PAA pure hydrogel (Fig. 3a) shows a relatively loose microstructure, which is intimately related to its covalent-cross-linked polymeric networks. With increasing the amount of GO, the fracture surfaces of the composite hydrogels (Fig. 3b–d) become compact gradually, which indicates the existence of intermolecular interactions between GO and the polymer chains. Furthermore, there appears to be a parallel-aligned fracture surface in the composite hydrogel with 0.30 wt% GO loading (Fig. 3c), which might be in accordance with the parallel-aligned interconnected pores observed with the optical microscope (Fig. 2). The similar fracture surface was also observed and explained in the GO/chitosan nanocomposite films, where the unidirectionally aligned GO sheets were in situ formed in the chitosan matrix, which enhanced its mechanical properties as well.27
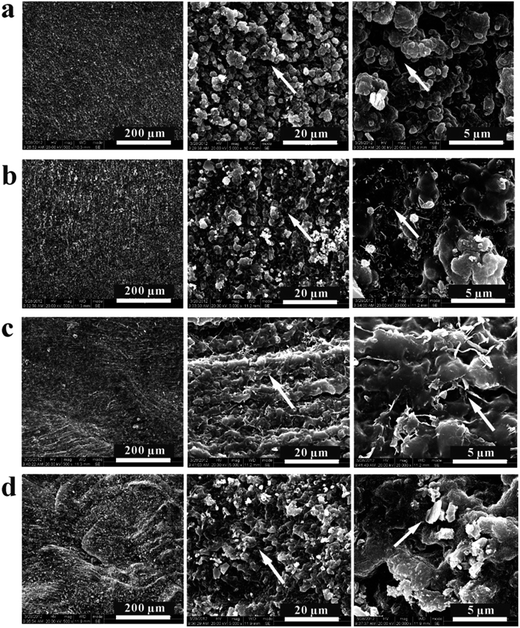 | ||
| Fig. 3 FESEM images with different magnifications of pure hydrogel (a) and composite hydrogels with 0.10 (b), 0.30 (c), and 0.50 (d) wt% GO loading in the dry state. | ||
WXRD is also an effective method to evaluate the exfoliation and dispersion of GO sheets in a polymer matrix by the interlayer spacing change of graphite oxide.18,19,37 As shown in Fig. S1,† compared with pristine graphite, the interlayer spacing of graphite oxide is increased from 0.336 to 0.817 nm, indicating the weakening of the interlayer van der Waals forces. Furthermore, the complete disappearance of the crystalline peak of graphite oxide in the composite hydrogel suggests that the exfoliated GO sheets are well dispersed in the matrix because of the effective intermolecular interaction, which is in agreement with the FESEM observation (Fig. 3).
Herein, we briefly explain the microstructure difference between pure and composite hydrogels in wet and dry states, as described in Fig. 2 and 3, respectively. For the CS-g-PAA pure hydrogel, because of its covalent hydrogel networks, the expended polymer chains just shrink to stacked polymer chains from the wet state to the dry state, and the interactions between the polymer chains are relatively weak. For the GO/CS-g-PAA composite hydrogels, however, the intermolecular interactions (e.g., hydrogen bonding or electrostatic attraction) between GO and the shrunk polymer chains might be further enhanced from the wet state to the dry state due to the existence of numerous oxygenated groups on the surface of GO sheets and the decrease in intermolecular distance. Thus, the composite hydrogels become much denser than the pure one after dewatering, as observed by FESEM in Fig. 3.
Additionally, we also aim to discuss the possible formation mechanism of the self-assembled macroporous composite hydrogels. As we mentioned previously, when dissolving chitosan into GO solution, the CS–GO combinations were self-assembled due to the effective intermolecular interactions. Because of the existence of adequate active –NH2 groups in the CS–GO units, it is easy for the monomer AA (containing an active –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– group) to be covalently grafted onto the CS molecular chains, which will be further confirmed by the FTIR result in the following discussion (Fig. 4a). After graft polymerization, it is assumed that the composite hydrogels should exhibit relatively dense networks at the local zone of CS–GO unit and relatively loose networks at the far zone of this unit. As shown in Fig. 2c (left), when the amount of GO is relatively low (<0.15 wt%), the porous hydrogels with relatively small pore sizes can be dominated by this active CS–GO unit. With increasing the amount of GO a little higher (0.15–0.30 wt%), the active CS–GO unit tends to form a bigger and relatively uniform “porous unit” (revealed in Fig. 2c, right) with the surrounding polymer chains. As a result, the porous hydrogels show relatively a larger and homogeneous pore size distribution.
C– group) to be covalently grafted onto the CS molecular chains, which will be further confirmed by the FTIR result in the following discussion (Fig. 4a). After graft polymerization, it is assumed that the composite hydrogels should exhibit relatively dense networks at the local zone of CS–GO unit and relatively loose networks at the far zone of this unit. As shown in Fig. 2c (left), when the amount of GO is relatively low (<0.15 wt%), the porous hydrogels with relatively small pore sizes can be dominated by this active CS–GO unit. With increasing the amount of GO a little higher (0.15–0.30 wt%), the active CS–GO unit tends to form a bigger and relatively uniform “porous unit” (revealed in Fig. 2c, right) with the surrounding polymer chains. As a result, the porous hydrogels show relatively a larger and homogeneous pore size distribution.
Therefore, GO/CS-g-PAA composite hydrogels with self-assembled macroporous structures were synthesized successfully by a facile strategy of in situ radical polymerization in this study, which provides a novel route for fabricating porous hydrogels. Based on the CS–GO combination units, the covalent and noncovalent intermolecular interactions between GO and the CS-g-PAA polymer networks should further occur to form the macroscopic-assembled composite hydrogels, which will be emphasized here.
Intermolecular interactions between GO and CS-g-PAA
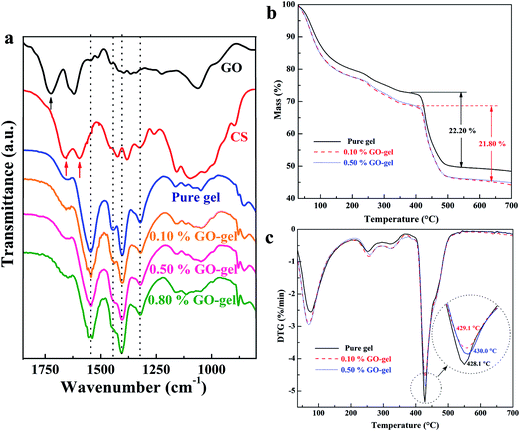
The FTIR spectra of GO, CS, CS-g-PAA hydrogel, and GO/CS-g-PAA composite hydrogels are shown in Fig. 4a. In the spectrum of GO, the absorption peaks at 1724, 1615, 1225, and 1060 cm−1 are characteristic of the –COOH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, –C–O–C–, and –C–OH groups respectively, which is in good agreement with the previous studies18,19 and proves the successful oxidation from graphite to graphite oxide. In the spectrum of CS, the absorption peaks at 1655, 1596, and 1060 cm−1 are related to the vibration of –C
C–, –C–O–C–, and –C–OH groups respectively, which is in good agreement with the previous studies18,19 and proves the successful oxidation from graphite to graphite oxide. In the spectrum of CS, the absorption peaks at 1655, 1596, and 1060 cm−1 are related to the vibration of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–NHCO–), –NH2, and –C–OH groups respectively.
O (–NHCO–), –NH2, and –C–OH groups respectively.
After graft polymerization, in the spectrum of CS-g-PAA hydrogel, the peak of –NH2 groups at 1596 cm−1 disappears, and the peaks of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–NHCO–) and –C–OH groups at 1655 and 1060 cm−1, respectively, become weak. Meanwhile, the absorption peaks at 1545 and 1403 cm−1 corresponding to the characteristics of the –COO− groups appear, indicating that the monomer AA has covalently grafted onto CS molecular chains successfully. Furthermore, the intermolecular interactions between GO and the CS-based polymer hydrogels are important for the self-assembled supramolecular structures and for the properties of the GO/CS-g-PAA composite hydrogels. Compared to the spectrum of CS-g-PAA pure hydrogel, some absorption peaks change in intensity or even disappear, and some new absorption peaks appear in the spectra of the GO/CS-g-PAA composite hydrogels, revealing the existence of effective intermolecular interactions between GO and the polymer chains. As the amount of GO increases, the absorption peak of the –COO− groups at 1545 cm−1 becomes broad visibly, and two shoulder peaks even appear at around 1545 cm−1 (for the 0.10 and 0.80 wt% GO gels), demonstrating that hydrogen bonds exist between GO and the polymer networks. In addition, in the spectra of the composite hydrogels, the absorption peak at 1724 cm−1 related to the –COOH groups of GO disappears, and the characteristic peak of the pure hydrogel at 1445 cm−1 becomes weak gradually, and even disappears with further increasing the GO loadings. These evidences suggest that covalent bonds might form between the –COOH groups of GO and the active groups (e.g. –NH2 or –OH groups) of the polymer networks. In fact, the reaction between GO and the polymers has also occurred in other GO/polymer composites.18,19,38 Therefore, as schematically demonstrated in Fig. 1b (right), the self-assembled supramolecular structures via effective covalent and noncovalent intermolecular interactions are probably formed in these GO-based composite hydrogels.
O (–NHCO–) and –C–OH groups at 1655 and 1060 cm−1, respectively, become weak. Meanwhile, the absorption peaks at 1545 and 1403 cm−1 corresponding to the characteristics of the –COO− groups appear, indicating that the monomer AA has covalently grafted onto CS molecular chains successfully. Furthermore, the intermolecular interactions between GO and the CS-based polymer hydrogels are important for the self-assembled supramolecular structures and for the properties of the GO/CS-g-PAA composite hydrogels. Compared to the spectrum of CS-g-PAA pure hydrogel, some absorption peaks change in intensity or even disappear, and some new absorption peaks appear in the spectra of the GO/CS-g-PAA composite hydrogels, revealing the existence of effective intermolecular interactions between GO and the polymer chains. As the amount of GO increases, the absorption peak of the –COO− groups at 1545 cm−1 becomes broad visibly, and two shoulder peaks even appear at around 1545 cm−1 (for the 0.10 and 0.80 wt% GO gels), demonstrating that hydrogen bonds exist between GO and the polymer networks. In addition, in the spectra of the composite hydrogels, the absorption peak at 1724 cm−1 related to the –COOH groups of GO disappears, and the characteristic peak of the pure hydrogel at 1445 cm−1 becomes weak gradually, and even disappears with further increasing the GO loadings. These evidences suggest that covalent bonds might form between the –COOH groups of GO and the active groups (e.g. –NH2 or –OH groups) of the polymer networks. In fact, the reaction between GO and the polymers has also occurred in other GO/polymer composites.18,19,38 Therefore, as schematically demonstrated in Fig. 1b (right), the self-assembled supramolecular structures via effective covalent and noncovalent intermolecular interactions are probably formed in these GO-based composite hydrogels.
It has been reported that the effective interactions can enhance the thermal property of polymers even with a very low amount of GO added.18,33,38 As shown in Fig. 4b and c, the thermal behavior of vacuum-dried CS-g-PAA hydrogel and GO/CS-g-PAA composite hydrogels was evaluated by TG and DTG methods. Both the pure and composite hydrogels decomposed mainly in a two-step process, and the composite hydrogels exhibited mildly enhanced thermal properties in the temperature range of 375–500 °C, compared to that of the pure one.
The first step of the weight loss from 30 to 375 °C was mainly attributed to the dewatering of the freeze-bonded and bonded water molecules, as well as the decomposition of the oxygenated functional groups in the hydrogel networks.16,21,22,39 From the TG curve of GO (Fig. S2†), it is easy to understand that the weight loss of the composite hydrogels is a little bit larger than that of pure hydrogel in this step, mainly because of the introduction of numerous oxygenated hydrophilic groups by the GO sheets. The second step of the weight loss from 375 to 500 °C was related to the thermal decomposition of the hydrogel networks. The parameters of the thermal decomposition behavior of pure and composite hydrogels, including the initial decomposition temperature (Ti), the temperature for the maximum decomposition rate (Tmax), the final decomposition temperature (Tf), and the maximum decomposition rate (Vdec) are also listed in Table S1.† It was found that the addition of a very low amount (≤0.50 wt%) of GO can improve the thermal property of dried hydrogels to some extent. In detail, the Ti, Tmax, and Tf of the composite hydrogel with 0.10 wt% GO loadings appeared at 417.7, 429.1, and 470.9 °C, and are increased by 4.0, 1.0, and 3.8 °C respectively, as compared to the pure hydrogel. In addition, the values of Vdec and the weight loss of this composite hydrogel are 4.58% min−1 and 21.80% respectively, which are slightly lower than that of the pure one. This enhancement of thermal property is also due to the fact that the active GO sheets can form effective intermolecular interactions with the polymer matrix, which was confirmed by the FESEM and FTIR results shown in Fig. 3 and 4a. Furthermore, the addition of GO might also increase the cross-linking density of hydrogel networks, resulting in the enhanced thermal property of the composite hydrogel. When the amount of GO was increased from 0.10 wt% to 0.50 wt%, however, the thermal property of the composite hydrogel was not improved obviously, probably because the partial aggregations of GO in the polymer networks are not beneficial for the improvement of thermal property.
Thereby, the above FTIR, TG, and DTG results further demonstrate that effective intermolecular interactions exist between GO sheets and the polymeric networks and are considered to be crucial for the formation of self-assembled macroporous composite hydrogels.
Swelling and mechanical properties of GO/CS-g-PAA composite hydrogels
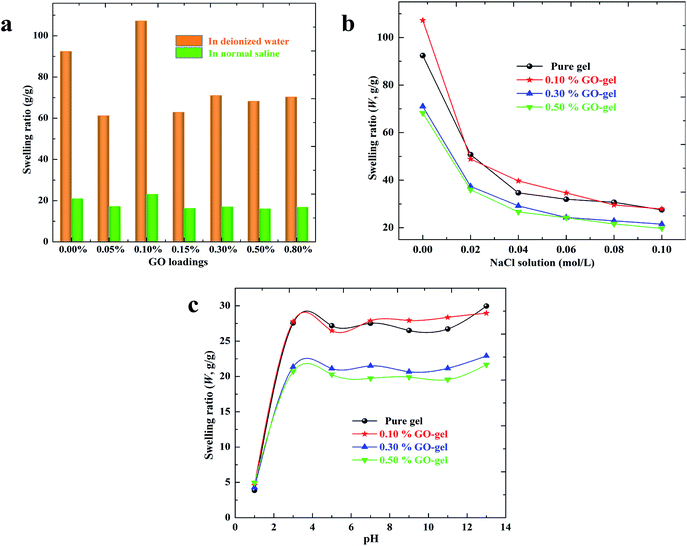
From the above discussion, it is clear that GO sheets can form interactions with the polymeric hydrogel networks through noncovalent and covalent bonding, and can significantly influence the microstructures of hydrogels as well. The incorporation of GO might also affect the swelling capacities of hydrogels. Meanwhile, the evaluation of the swelling capacities of hydrogels in aqueous solutions is critically essential for both a fundamental understanding and for practical applications. As shown in Fig. 5a, the swelling ratios of both pure and composite hydrogels in normal saline decline sharply, compared to that in deionized water. Despite the existence of a self-assembled structure due to the introduction of GO sheets, the composite hydrogels are mainly structured by covalent bonds and hydrogen bonds, as illustrated in Fig. 2b. Therefore, the above significant difference of composite hydrogels in swelling ratios between them being in water and in normal saline can still be explained as a common phenomenon in the swelling of polyelectrolyte hydrogels.18,34 This phenomenon is due to the fact that the charge screening effect of counter-ions (Na+ or Cl−) on the charged groups (–COO− or –NH3+) in the hydrogel networks can induce a clear decline of electrostatic repulsions, resulting in a decrease in the osmotic pressure between the hydrogel networks and external solution.Several aspects might affect the swelling ratios of GO/CS-g-PAA composite hydrogels, such as the intermolecular interactions between the components, the abundant hydrophilic groups on the surface of GO, the microstructures of hydrogels, and the dispersion of GO in the matrix. It can be found that the hydrogels exhibit a similar swelling tendency in both media with increasing the amount of GO. Compared with the pure hydrogel, the swelling ratios of composite hydrogel with 0.05 wt% GO loading decreased mildly, indicating that the intermolecular interactions between the components restrict the swelling of hydrogel networks. When increasing the amount of GO from 0.05 to 0.10 wt%, the composite hydrogels acquired evidently increased swelling ratios. The enhanced swelling capacities might be due to the influence of GO, which contains numerous hydrophilic groups, which can remarkably increase the density of hydrophilic groups in the hydrogel networks. Furthermore, GO sheets dispersed homogeneously in the matrix can probably improve the swelling capacities as well. However, with further increasing the amount of GO, the swelling ratios of composite hydrogels decrease apparently. This might be attributed to the further increased cross-linking density of the composite hydrogel networks and the aggregations of excessive GO sheets in the matrix, which was confirmed by the FESEM morphologies, as shown in Fig. 3.
According to Flory's swelling theory,40 the swelling behaviors of hydrogels are strongly affected by the ionic strength of external solutions. Additionally, the prepared hydrogels might also exhibit different swelling behaviors in a wide range of pH values, since both pure and composite hydrogels contain charged groups (–COOH and –NH2 groups), usually called polyelectrolyte hydrogels. As shown in Fig. 5b and c, the effects of salt concentration and pH value of the media on the swelling ratios of hydrogels are presented, and are discussed as follows. It can be observed that both pure and composite hydrogels show a similar swelling tendency in salt and pH solutions respectively, indicating that GO sheets have very little influence on the salt- and pH-sensitive behaviors of hydrogel networks.
As aforementioned, the swelling decline of polyelectrolyte hydrogels in salt solutions is often attributed to a charge screening effect of counter-ions, which can significantly weaken the ion–ion electrostatic repulsion among the hydrogel networks.18,34 The effects of salt concentration on the swelling ratios of hydrogels, evaluated in NaCl solutions, are shown in Fig. 5b. The swelling ratios of hydrogels decrease with increasing the salt concentration of NaCl solution due to the increased charge screening effect of counter-ions and the decreased osmotic pressure between the hydrogel networks and the external solution, which is in good accord with Donnan equilibrium theory.
From the swelling ratios of hydrogels in pH solutions (Fig. 5c), it can be seen that all hydrogels exhibit a clear pH-sensitive swelling capability. It is noted that most –NH2 groups on chitosan chains have been reacted and thereby consumed by the graft polymerization. Therefore, the variation in the swelling capability is mainly attributed to the changes in the protonation of the carboxylic groups on the PAA chains according to the pH variations. All the hydrogels acquired relatively low swelling ratios at very acidic media (pH < 3) due to the strong protonation of –COOH groups in the hydrogel networks. In this case, a great number of hydrogen bonds formed among –COOH, –OH, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and greatly restrict the swelling of the hydrogel despite the existence of slight NH3+–NH3+ electrostatic repulsion in the hydrogel networks, leading to the relatively low swelling ratios. As the pH value of buffer solution increases, the swelling ratios of the hydrogels increase sharply before maintaining a relatively stable value. This phenomenon results from the fact that numerous –COOH groups in the hydrogel networks are ionized and converted into –COO− groups, so that many hydrogen bonds are broken and the sufficient COO−–COO− electrostatic repulsion increases remarkably among the hydrogel networks. This effect causes the hydrogel matrix to expand, and hence the amount of liquid absorbed increases considerably. The above swelling behavior in different pH solutions is well in agreement with the previous study on chitosan-graft-poly(acrylic acid) hydrogels.41 Meanwhile, when the amount of GO increases to 0.30 wt% and 0.50 wt%, the swelling ratios of composite hydrogels decrease slightly in both the salt and pH solutions, compared to that of the pure one. This result might be also owing to the fact that the enhanced interactions between GO and polymer chains and the aggregation of GO in the matrix do not benefit the swelling of hydrogel networks.
O groups and greatly restrict the swelling of the hydrogel despite the existence of slight NH3+–NH3+ electrostatic repulsion in the hydrogel networks, leading to the relatively low swelling ratios. As the pH value of buffer solution increases, the swelling ratios of the hydrogels increase sharply before maintaining a relatively stable value. This phenomenon results from the fact that numerous –COOH groups in the hydrogel networks are ionized and converted into –COO− groups, so that many hydrogen bonds are broken and the sufficient COO−–COO− electrostatic repulsion increases remarkably among the hydrogel networks. This effect causes the hydrogel matrix to expand, and hence the amount of liquid absorbed increases considerably. The above swelling behavior in different pH solutions is well in agreement with the previous study on chitosan-graft-poly(acrylic acid) hydrogels.41 Meanwhile, when the amount of GO increases to 0.30 wt% and 0.50 wt%, the swelling ratios of composite hydrogels decrease slightly in both the salt and pH solutions, compared to that of the pure one. This result might be also owing to the fact that the enhanced interactions between GO and polymer chains and the aggregation of GO in the matrix do not benefit the swelling of hydrogel networks.
From the above discussion, the addition of GO sheets only shows mild effects on the swelling behavior in aqueous fluids. However, GO sheets can evidently enhance the mechanical property of composite hydrogels. As shown in Fig. 6, the compressive performance of the wet composite hydrogel with 0.50 wt% GO loading exhibits a significant reinforcement compared to that of the pure one. The pure hydrogel is very brittle and easily destroyed under compressive load. Despite a high water content (CW > 98 wt%, similar to the pure hydrogel, as presented in Fig. S3†), the composite hydrogels are relatively tough, and only show a very small strain under a constant load. It is noted that the composite hydrogels can recover their original shapes immediately after removing the load. This reinforcement is mainly attributed to both the unique mechanical nature of GO sheets and the intermolecular interactions between GO and the polymer chains, which are beneficial for the effective load transfer in the hydrogel networks and lead to the enhanced load-bearing ability. In fact, a similar mechanical reinforcement has also been evidenced in other GO/polymer composite hydrogel systems.19,37
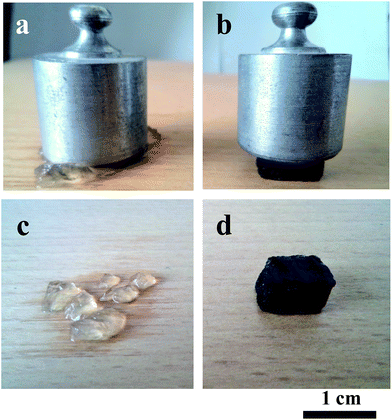 | ||
| Fig. 6 Photographs of pure hydrogel (a and c) and composite hydrogel (b and d) during and after compressed condition. The weight of load is 100 g. | ||
Therefore, the GO/CS-g-PAA composite hydrogels possess both macroporous structures and enhanced mechanical performance, yet can still hold similar swelling properties from their parent CS-g-PAA hydrogel. Composite hydrogels with these combined properties will probably demonstrate an enhanced adsorption capability to heavy metal ions or organic dyes in wastewater treatment. Of course, the nature and practical applications of the porous structures for GO-based composite hydrogels require further investigation.
Conclusions
In conclusion, we have developed a series of self-assembled GO/CS-g-PAA macroporous composite hydrogels via a facile radical polymerization. Due to the adequate hydrophilic oxygenated groups on the surface, GO sheets can be dispersed well and can form self-assembled supramolecular structures with polymer chains by effective intermolecular interactions, such as hydrogen bonding, electrostatic attraction, or covalent bonding. An extremely low amount (0.05–0.30 wt%) of GO can remarkably affect the architecture of CS-g-PAA hydrogel networks, leading to the formation of macroporous composite hydrogels. The self-assembled graphene oxide–chitosan units are assumed to play a critical role in the structure formation of composite hydrogels. It is worth noting that this study provides a simple path for fabricating porous hydrogels. In addition, the addition of GO sheets shows a slight influence on the swelling behavior of hydrogels in various media, and the composite hydrogels exhibit salt- and pH-sensitivities, similar to the pure one. Thanks to the effective intermolecular interactions between GO and the hydrogel matrix, the composite hydrogels also demonstrate evidently enhanced mechanical performance, as compared to that of the pure hydrogel matrix. Thus, the self-assembled macroporous composite hydrogels could find some potential applications in wastewater treatment or biomedical engineering. Our upcoming study is going to focus on the comprehensive characterization of the mechanical properties of composite hydrogels and experiments for their practical applications.Acknowledgements
We gratefully thank the SRF for ROCS, State Education Ministry, PR China, the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (Contract Grant No. CUGL090223), Hubei Provincial Department of Education (XD2010037), the grant of the Opening Project of State Key Laboratory of Polymer Materials Engineering (Sichuan University) (KF201106) and Engineering Research Center of Nano-Geomaterials of Ministry of Education (CUG). This study is partially supported by National High-Tech R&D Program (863 program) for the 12th Five-Year Plan, Ministry of Science and Technology, PR China (SQ2010AA1000690005).Notes and references
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- J. Kopeček, Biomaterials, 2007, 28, 5185–5192 CrossRef PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef CAS PubMed.
- A. Gregorova, N. Saha, T. Kitano and P. Saha, Carbohydr. Polym., 2015, 117, 559–568 CrossRef CAS PubMed.
- M. A. Zwieniecki, P. J. Melcher and N. M. Michele Holbrook, Science, 2001, 291, 1059–1062 CrossRef CAS PubMed.
- Y. Chen, L. Chen, H. Bai and L. Li, J. Mater. Chem. A, 2013, 1, 1992 CAS.
- N. Kato and S. H. Gehrke, Colloids Surf., B, 2004, 38, 191–196 CrossRef CAS PubMed.
- A. Barbetta, G. Rizzitelli, R. Bedini, R. Pecci and M. Dentini, Soft Matter, 2010, 6, 1785 RSC.
- V. Gopishetty, I. Tokarev and S. Minko, J. Mater. Chem., 2012, 22, 19482 RSC.
- A. Harada, R. Kobayashi, Y. Takashima, A. Hashidzume and H. Yamaguchi, Nat. Chem., 2011, 3, 34–37 CrossRef CAS PubMed.
- H. Park, A. Afzali, S.-J. Han, G. S. Tulevski, A. D. Franklin, J. Tersoff, J. B. Hannon and W. Haensch, Nat. Nanotechnol., 2012, 7, 787–791 CrossRef CAS PubMed.
- A. Hernandez-Garcia, D. J. Kraft, A. F. J. Janssen, P. H. H. Bomans, N. A. J. M. Sommerdijk, D. M. E. Thies-Weesie, M. E. Favretto, R. Brock, F. A. de Wolf, M. W. T. Werten, P. van der Schoot, M. C. Stuart and R. de Vries, Nat. Nanotechnol., 2014, 9, 698–702 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- W. Chen and L. Yan, Nanoscale, 2011, 3, 3132–3137 RSC.
- W. Ai, Z.-Z. Du, J.-Q. Liu, F. Zhao, M.-D. Yi, L.-H. Xie, N.-E. Shi, Y.-W. Ma, Y. Qian, Q.-L. Fan, T. Yu and W. Huang, RSC Adv., 2012, 12204–12209 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- Y. Huang, M. Zeng, J. Ren, J. Wang, L. Fan and Q. Xu, Colloids Surf., A, 2012, 401, 97–106 CrossRef CAS.
- H. P. Cong, P. Wang and S. H. Yu, Chem. Mater., 2013, 25, 3357–3362 CrossRef CAS.
- A. Pourjavadi, M. Nazari and S. H. Hosseini, RSC Adv., 2015, 5, 32263–32271 RSC.
- H. Zhang, X. Pang and Y. Qi, RSC Adv., 2015, 5, 89083–89091 RSC.
- H. Zhang, D. Zhai and Y. He, RSC Adv., 2014, 4, 44600–44609 RSC.
- Y. Wang, P. Zhang, C. F. Liu and C. Z. Huang, RSC Adv., 2013, 3, 9240 RSC.
- H. Bai, C. Li, X. Wang and G. Shi, Chem. Commun., 2010, 46, 2376 RSC.
- Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS PubMed.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS.
- Y. Pan, T. Wu, H. Bao and L. Li, Carbohydr. Polym., 2011, 83, 1908–1915 CrossRef CAS.
- G. Z. Kyzas, D. N. Bikiaris, M. Seredych, T. J. Bandosz and E. A. Deliyanni, Bioresour. Technol., 2014, 152, 399–406 CrossRef CAS PubMed.
- X. Yang, Y. Tu, L. Li, S. Shang and X. M. Tao, ACS Appl. Mater. Interfaces, 2010, 2, 1707–1713 CAS.
- A. Ray Chowdhuri, S. Tripathy, S. Chandra, S. Roy and S. K. Sahu, RSC Adv., 2015, 5, 49420–49428 RSC.
- D. Han and L. Yan, ACS Sustainable Chem. Eng., 2014, 2, 296–300 CrossRef CAS.
- G. R. Mahdavinia, A. Pourjavadi, H. Hosseinzadeh and M. J. Zohuriaan, Eur. Polym. J., 2004, 40, 1399–1407 CrossRef CAS.
- M. Zeng, J. Wang, R. Li, J. Liu, W. Chen, Q. Xu and Y. Gu, Polymer, 2013, 54, 3107–3116 CrossRef CAS.
- C. Chang, M. He, J. Zhou and L. Zhang, Macromolecules, 2011, 44, 1642–1648 CrossRef CAS.
- S. Bhattacharyya, S. Guillot, H. Dabboue, J. F. Tranchant and J. P. Salvetat, Biomacromolecules, 2008, 9, 505–509 CrossRef CAS PubMed.
- D. Ceylan and O. Okay, Macromolecules, 2007, 40, 8742–8749 CrossRef CAS.
- L. Zhang, Z. Wang, C. Xu, Y. Li, J. Gao, W. Wang and Y. Liu, J. Mater. Chem., 2011, 21, 10399 RSC.
- S. Sun and P. Wu, J. Mater. Chem., 2011, 21, 4095 RSC.
- Z. X. Tai, J. Yang, Y. Y. Qi, X. B. Yan and Q. J. Xue, RSC Adv., 2013, 3, 12751–12757 RSC.
- P. J. Flory, Principles of polymer chemistry, 1953, pp. 1–672 Search PubMed.
- F. H. A. Rodrigues, A. G. B. Pereira, A. R. Fajardo and E. C. Muniz, J. Appl. Polym. Sci., 2013, 128, 3480–3489 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The WXRD data of GO-based composite hydrogels (Fig. S1), TG data of graphene oxide (Fig. S2), and TG/DTG data (Table S1) and water content (Fig. S3) of pure hydrogel and GO-based composite hydrogels. See DOI: 10.1039/c5ra25910j |
| ‡ Present address: Graduate School of Life Science, Hokkaido University, Sapporo 060-0810, Japan. |
| This journal is © The Royal Society of Chemistry 2016 |