Modeling of thermal conductivity of nanofluids considering aggregation and interfacial thermal resistance
Abstract
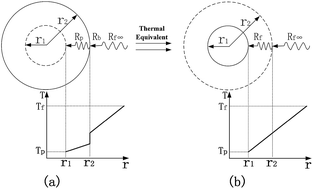
A model with consideration given to particle size, aggregate size and interfacial thermal resistance was developed to predict the thermal conductivity of nanofluids. Interfacial thermal resistance was modeled and found to have a relationship with the equivalent particle size, in terms of keeping the thermal resistance constant. The shape factor of the aggregate was determined by the number of particles in the aggregate. The presented model agrees well with the widely accepted experimental data. It was concluded that particle size and aggregate size have positive effects on the thermal conductivity enhancement, because the increase of particle size can weaken the effect of interfacial thermal resistance, and the increase of aggregate size can offer a fast heat transfer path for adjacent particles and it significantly increases the shape factor of the aggregate. The thermal conductivity of nanofluids increases linearly with particle volume fraction and the increase rate differs according to particle size and aggregate size. The inferred values of interfacial thermal resistance are in a reasonable range and fit well with different experimental data. If the particle volume fraction is lower than 0.1%, or if the particle size is smaller than 10 nm without aggregation, the factors of nano-convection and nanolayers need to be taken into account.

 Please wait while we load your content...
Please wait while we load your content...