Greener technology for organic reactive dye degradation in textile dye-contaminated field soil and in situ formation of “electroactive species” at the anode by electrokinetics†
Abstract
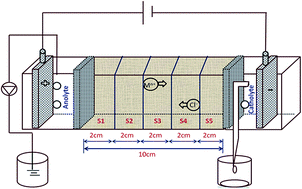
The present study focuses on the electrokinetic process for the in situ formation of electroactive species at the anode. A bench scale experiment was conducted to investigate the organic molecule degradation by in situ electroactive species and simultaneous removal of trace metals in soil contaminated by a textile effluent. With an impressed direct current (DC), the voltage gradient of 2 V cm−1 facilitated the dye/metal ion movement in the contaminated soil towards the electrodes by electromigration. Two different electrolytes were evaluated for the degradation of pollutants in the soil. Thus, 0.5 M potassium sulfate was assayed as the anolyte and catholyte for system I and in system II, distilled water was used as the electrolyte. The EK process experiments were conducted for 120 h. The UV-Visible spectrum revealed the presence of humic substances in the anolyte chamber during the EK process. The EK treatment was accomplished by the in situ anodic generation of electroactive species. These species were able to degrade the organic components efficiently. The formation of peroxide was noticed in the range of 18 to 32 mg L−1 (system I) during the anodic EK processes. After the EK process, the total organic content was reduced by about 68% and 41% for system I and system II, respectively, which clearly indicates that the significant removal of organic content in system I as compared to that in system II. The trace metals nickel, chromium and copper were significantly removed from the contaminated soil. This technology may be exploited for a faster and eco-friendly degradation and removal of textile dye and inorganic substances in the soil environment.

 Please wait while we load your content...
Please wait while we load your content...