Resonance Rayleigh scattering and resonance nonlinear scattering methods for the determination of nicardipine hydrochloride using eosin Y as a probe
Abstract
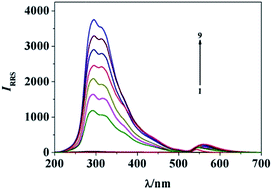
In a Britton–Robinson (pH 3.5) buffer solution, nicardipine hydrochloride (NCD) reacted with eosin Y (EY) to form an ion-association complex of [NCD·EY], which would self-aggregate to form [NCD·EY]n nanoparticles with an average size of 55 nm via the squeezing effect of the aqueous phase and the van der Waals force. As a result, the intensities of resonance Rayleigh scattering (RRS), second-order scattering (SOS) and frequency doubling scattering (FDS) were enhanced and the new scattering spectra appeared. The maximum RRS, SOS and FDS wavelengths were located at 294 nm, 552 nm and 327 nm, respectively. The increments of scattering intensity (ΔIRRS, ΔISOS and ΔIFDS) were directly proportional to the concentration of NCD in certain ranges. The detection limits (3σ) of RRS, SOS and FDS were 1.3 ng mL−1, 2.2 ng mL−1 and 1.6 ng mL−1. The optimum conditions of the RRS method and the influence factors were discussed. In addition, the structure of the ion-association complex and the reaction mechanism were investigated. The shape of nanoparticles was characterized by transmission electron microscopy. The reaction mechanism and the reasons for enhancement of scattering were discussed based on infrared spectra, quantum chemical calculations and absorption spectroscopy. Accordingly, a novel rapid, convenient, sensitive and selective RRS method for determination of NCD was proposed and applied to detect NCD in tablet and urine samples with satisfactory results.

 Please wait while we load your content...
Please wait while we load your content...