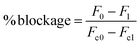New transient receptor potential TRPV1, TRPM8 and TRPA1 channel antagonists from a single linear β,γ-diamino ester scaffold†
Paula Pérez-Faginasa,
M. Teresa Arandaa,
Roberto de la Torre-Martínezb,
Susana Quirceb,
Asia Fernández-Carvajalb,
Antonio Ferrer-Montielb and
Rosario González-Muñiz*a
aInstituto de Química Médica (IQM-CSIC), Juan de la Cierva 3, 28006 Madrid, Spain. E-mail: rosario.gonzalezmuniz@iqm.csic.es
bInstituto de Biología Molecular y Celular, Universidad Miguel Hernández, Avenida de la Universidad s/n, 03202 Elche, Alicante, Spain
First published on 12th January 2016
Abstract
A high throughput screening campaign identified nine β,γ-diamino ester derivatives as TRP modulators. A discrete library of new derivatives (23 components) was prepared in a one-pot two step reductive amination reaction, and evaluated for their ability to block the agonist-induced calcium influx in cells expressing human TRPV1, TRPM8 and TRPA1 channels. Selective antagonists for each channel, as well as dual TRPV1/TRPM8 and TRPM8/TRPA1 ligands, were obtained after subtle modification of this linear scaffold. SAR studies revealed the preferred substituents for the selective blockade of the three TRP channels under study. The most potent TRPV1 antagonists displayed submicromolar IC50 values.
Introduction
Membrane transient receptor potential (TRP) proteins are voltage- and ligand-gated ion channels with a variety of biological functions and are apparently implicated in diverse pathological conditions. According to sequence similarities, mammalian TRP channels comprise six subfamilies with low sequence identity among them (as low as 20%), named TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPV (vanilloid).1A few members of the TRP family (TRPV1-4, TRPM8 and TRPA1), belonging to three different subfamilies, are the so-called thermoTRP channels, which participate in the detection of temperature changes and also integrate different noxious stimuli.2 Thus, TRPV1 is a non-selective Ca2+ channel activated by noxious temperatures (>43 °C), acidic pH and vanilloid compounds. TRPV1 expression is overregulated under acute inflammatory states3–5 and in chronic pain conditions,6 and its activity is potentiated by proalgesic mediators after inflammation and tissue injury.7,8 TRPM8 channels have a physiological role in detecting low temperature (10–33 °C),9 and are also over-expressed in sensory neurons after nerve injury or inflammation,10 as well as involved in cold allodynia and hyperalgesia.11 TRPA1 is also a non-selective Ca2+ channel activated by multiple stimuli, including harmful cold temperatures, acids, and numerous chemical pollutants.12–15 TRPA1 receptors are coexpressed with TRPV1 channels in C-fiber sensory neurons,16 and seems to have a crucial role in neuronal and non-neuronal neuropathic pain.17–19
Since patients with inflammatory or neuropathic pain suffer from hypersensitivity to mechanical, thermal and/or chemical stimuli, an approach to develop successful analgesic therapies may be to target TRPV1, TRPM8 and TRPA1 nociceptors.20 However, despite the big number of compounds currently available in this field, their poor specificity and side effects justify the need for new compounds.21 A few representative modulators of these three types of TRP channels are depicted in Chart 1. Knowledge about the molecular requirements that makes a particular family of compounds to bind to one or more of these channels is still scarce and, in general, the described compounds for every TRP channel are structurally very different among them.21
Within our programs of innovative chemical libraries we have recently described a series of β,γ-diamino ester derivatives 1–4 as valuable building block intermediates to heterocyclic compounds (Chart 2).22 Following our interest in TRP modulators,23–26 some of these derivatives were evaluated in our HTS screening platform for the search of new TRP channel ligands. Within them, we found either selective antagonists for TRPV1 and TRPM8 or dual TRPM8/TRPA1 blockers, although they are very closely related compounds. To explore further the interest of these β,γ-diamino ester derivatives as TRP ligands, and to look deeply into the structural particularities behind the TRP channel type preferences, in this paper we describe the results of the biological evaluation of compounds 1–4, along with the preparation and HTS screening of new derivatives within this series.
Results and discussion
Chemistry
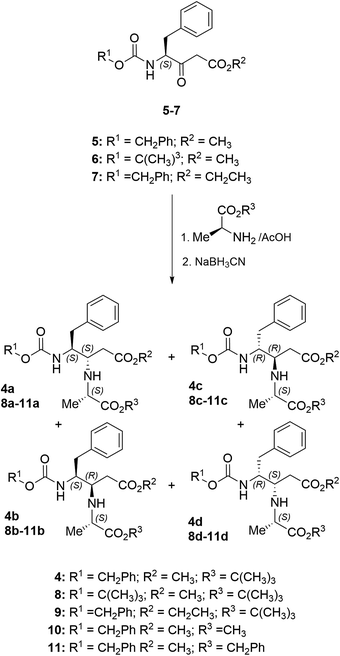
To understand the role of the benzyloxycarbonyl (Z) and ester groups in 4, we first aim to prepare the Boc analogue 8 and some ester variations, 9–11. Highly functionalized β,γ-diamino esters 8–11, derived from Phe and Ala, were prepared following our previously described method for analogues 4a–c.22 Briefly, the reaction of β-ketoesters 5–7 with the corresponding Ala-derived α-amino esters in the presence of AcOH led to imine intermediates, which were then reduced with NaBH3CN. This two-step reductive amination procedure afforded compounds 4, 8–11 as mixtures of diastereoisomers that, in most cases, can be separated by column chromatography. As described, three isomers had been isolated for compound 4a–c, and their configurations had indirectly been determined through transformation to pyrrolidinone derivatives.22 Similarly, the formation of three major stereoisomers was observed in the case of compound 8–10, where only traces of a fourth diastereoisomer could be detected by HPLC-MS (not isolated). However, for the Ala-OBn derivative 11, all four possible diastereoisomers were formed, although isomers 11c and 11d could not be separated in pure form. In each case the configurational assignment was performed by comparison of NMR and HPLC data with those of 4a–c (Scheme 1).The loss of the stereochemical integrity of the initial amino acid-derived β-ketoester, leading to diastereoisomers c and d, could be explained through the existence of imine–enamine tautomerism, as previously determined by reduction with NaBD3CN.22
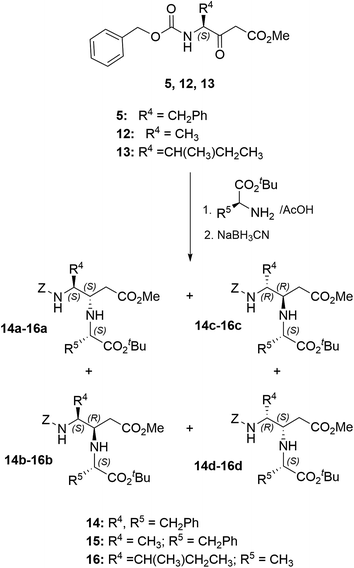
To set light on the importance of the starting amino acids side chains, we then prepared compounds 14–16, in which the initial Ala-OtBu derivative was changed by Phe-OtBu, and the Z-Phe-derived ketoester was substituted by those resulting from Z-Ala and Z-Ile (Scheme 2). Phe–Phe-derived compound 14 was obtained as a 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixture of a–d diastereoisomers, with 14c–14d isolated as an inseparable mix. However, three isomers were detected for the Ala–Phe analogue 15, although only isomers 15a and 15b were separated in pure form. Four main isomers were obtained in the case of compound 16, from which isomers a, b and d could be totally separated. As the Ile residue has an additional stereogenic center, other minor isomers were detected by HPLC and in certain NMR fractions (not isolated). As indicated above, the configuration assignment was tentatively done by comparison with isomers 4a–c.
10 mixture of a–d diastereoisomers, with 14c–14d isolated as an inseparable mix. However, three isomers were detected for the Ala–Phe analogue 15, although only isomers 15a and 15b were separated in pure form. Four main isomers were obtained in the case of compound 16, from which isomers a, b and d could be totally separated. As the Ile residue has an additional stereogenic center, other minor isomers were detected by HPLC and in certain NMR fractions (not isolated). As indicated above, the configuration assignment was tentatively done by comparison with isomers 4a–c.
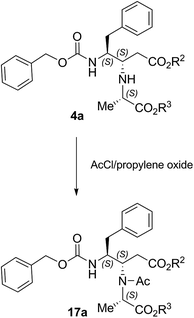
Finally, to explore the importance of the 3-NH group, we prepare the corresponding acetyl derivative 17a by treatment of compound 4a with acetyl chloride in the presence of propylene oxide as HCl scavenger (Scheme 3).
Biological evaluation
All compounds were assayed on TRPV1, TRPM8 and TRPA1 channels, stably expressed in the appropriate cell lines (see Experimental for details). The agonist-induced intracellular Ca2+ signals were measured by microfluorography, using a fluorescence plate reader, in the absence and in the presence of test compounds. Capsaicin (TRPV1), menthol (TRPM8) and allylisothiocyanate (TRPA1) were used as the respective agonists. The obtained results were compared toward those of AMTB (TRPM8 antagonist) and ruthenium red (TRPV1, TRPA1 unspecific antagonist). The obtained results are recorded in Tables 1 and 2.| Compd | Config. 3,4 or 3,4,1′ | R | Concentration (μM) | TRPV1a | TRPM8b | TRPA1c |
|---|---|---|---|---|---|---|
| a Blockade of Ca2+ entry through TRPV1 channel by compounds (capsaicin, 10 μM, was used as the agonist).b Blockade of Ca2+ entry through TRPM8 channel by compounds (menthol, 100 μM, was used as the agonist).c Blockade of Ca2+ entry through TRPA1 channel by compounds (allyl isothiocyanate, 100 μM, was used as the agonist).d Compounds were assayed at 50 μM (up) and 5 μM (down) concentrations. NA: blockade lower than 25% at the higher concentration assayed. | ||||||
| 1a | S,S | CH2Ph | 50 | 31.50 ± 16.63 | 89.08 ± 5.40 | NA |
| 5 | 29.48 ± 6.46 | 57.54 ± 1.67 | ||||
| 1b | R,S | CH2Ph | 50 | 65.05 ± 2.72 | 91.89 ± 4.07 | NA |
| 5 | 14.60 ± 24.26 | 60.66 ± 5.34 | ||||
| 2a | S,S | (CH2)3CH3 | 50 | 89.68 ± 11.88 | 74.93 ± 3.11 | NA |
| 5 | 21.11 ± 8.00 | 52.98 ± 6.46 | ||||
| 2b | R,S | (CH2)3CH3 | 50 | 54.95 ± 3.51 | 83.91 ± 4.42 | NA |
| 5 | 15.14 ± 7.65 | 56.79 ± 6.11 | ||||
| 3a | S,S | CH2CO2Me | 50 | 57.38 ± 3.59 | 84.36 ± 5.41 | 80.14 ± 14.67 |
| 5 | 22.00 ± 3.51 | 37.83 ± 11.03 | 70.34 ± 13.25 | |||
| 3b | R,S | CH2CO2Me | 50 | 84.22 ± 9.08 | 79.51 ± 4.71 | 60.86 ± 10.71 |
| 5 | 24.46 ± 15.73 | 36.96 ± 14.30 | 66.87 ± 17.04 | |||
| 4a | S,S,S | — | 50 | 99.19 ± 12.10 | NA | NA |
| 5 | 73.50 ± 9.62 | |||||
| 4b | R,S,S | — | 50 | 109.28 ± 6.54 | 62.29 ± 10.46 | NA |
| 5 | 72.25 ± 11.10 | −15.13 ± 19.57 | ||||
| 4c | R,R,S | — | 50 | 106.76 ± 9.82 | 57.45 ± 22.47 | NA |
| 5 | 49.91 ± 22.57 | −8.20 ± 17.97 | ||||
| Ruthenium red | — | 10 | 100% | 100% | ||
| AMTB | — | 10 | 100% | |||
| Compd | Config. 3,4,1′ | R1 | R2 | R3 | R4 | R5 | TRPV1a | TRPM8b | TRPA1c |
|---|---|---|---|---|---|---|---|---|---|
| a Blockade of Ca2+ entry through TRPV1 channel by compounds (capsaicin, 10 μM, was used as the agonist).b Blockade of Ca2+ entry through TRPM8 channel by compounds (menthol, 100 μM, was used as the agonist).c Blockade of Ca2+ entry through TRPA1 channel by compounds (allyl isothiocyanate, 100 μM, was used as the agonist).d Compounds were assayed at 50 μM (up) and 5 μM (down) concentrations. NA: blockade lower than 25% at the higher concentration assayed. | |||||||||
| 8a | S,S,S | Boc | Me | tBu | Bn | Me | 106.76 ± 9.82 | 88.55 ± 13.60 | NA |
| 52.12 ± 12.39 | 14.64 ± 8.42 | ||||||||
| 8b | R,S,S | Boc | Me | tBu | Bn | Me | 103.46 ± 8.27 | 33.14 ± 21.05 | NA |
| 62.00 ± 10.03 | 9.56 ± 2.95 | ||||||||
| 8c | R,R,S | Boc | Me | tBu | Bn | Me | 89.97 ± 2.62 | 38.96 ± 13.62 | NA |
| 49.45 ± 4.86 | 8.34 ± 11.67 | ||||||||
| 9a | S,S,S | Z | Et | tBu | Bn | Me | 88.30 ± 8.70 | 85.72 ± 5.01 | NA |
| 74.58 ± 5.24 | 70.77 ± 5.49 | ||||||||
| 9b | S,R,S | Z | Et | tBu | Bn | Me | 95.83 ± 5.81 | 87.90 ± 6.25 | NA |
| 72.35 ± 17.12 | 39.78 ± 7.97 | ||||||||
| 9c | R,R,S | Z | Et | tBu | Bn | Me | 92.95 ± 12.14 | 96.97 ± 6.91 | NA |
| 50.49 ± 16.58 | 40.51 ± 8.85 | ||||||||
| 10a | S,S,S | Z | Me | Me | Bn | Me | 75.53 ± 1.24 | 57.83 ± 5.92 | NA |
| 23.74 ± 15.03 | 13.48 ± 7.33 | ||||||||
| 10b | R,S,S | Z | Me | Me | Bn | Me | 28.41 ± 12.41 | 51.83 ± 8.30 | NA |
| 9.65 ± 11.52 | 16.14 ± 16.27 | ||||||||
| 10c | R,R,S | Z | Me | Me | Bn | Me | NA | 46.54 ± 7.18 | NA |
| 19.92 ± 7.52 | |||||||||
| 11a | S,S,S | Z | Me | Bn | Bn | Me | NA | 96.89 ± 2.67 | 93.46 ± 9.16 |
| 69.79 ± 6.29 | 67.07 ± 7.47 | ||||||||
| 11b | R,S,S | Z | Me | Bn | Bn | Me | 42.49 ± 6.83 | 96.68 ± 3.01 | 90.61 ± 15.78 |
| 17.92 ± 1.74 | 43.10 ± 8.24 | 67.84 ± 15.94 | |||||||
| 11c,d | R,R,S | Z | Me | Bn | Bn | Me | 63.56 ± 5.18 | 95.91 ± 4.66 | 90.05 ± 18.55 |
| S,R,S | 24.24 ± 7.06 | 65.65 ± 3.47 | 71.53 ± 4.30 | ||||||
| 14a | S,S,S | Z | Me | tBu | Bn | Bn | 67.05 ± 9.43 | 102.85 ± 3.00 | 57.07 ± 18.64 |
| 15.16 ± 12.92 | 73.47 ± 7.41 | 44.20 ± 16.94 | |||||||
| 14b | R,S,S | Z | Me | tBu | Bn | Bn | 90.77 ± 2.17 | 86.91 ± 3.09 | 78.14 ± 9.53 |
| 45.92 ± 14.74 | 47.09 ± 5.84 | 60.18 ± 11.21 | |||||||
| 14c,d | R,R,S | Z | Me | tBu | Bn | Bn | 85.37 ± 5.86 | 92.96 ± 4.22 | 81.99 ± 9.40 |
| S,R,S | 42.17 ± 8.30 | 40.50 ± 13.24 | 54.25 ± 11.60 | ||||||
| 15a | S,S,S | Z | Me | tBu | Me | Bn | 54.46 ± 8.92 | 86.74 ± 5.26 | NA |
| 17.90 ± 9.01 | 34.81 ± 5.85 | ||||||||
| 15b | R,S,S | Z | Me | tBu | Me | Bn | 91.09 ± 3.78 | 86.35 ± 6.97 | NA |
| 31.40 ± 4.84 | 29.10 ± 22.35 | ||||||||
| 16b | R,S,S | Z | Me | tBu | s-Bu | Me | 61.88 ± 11.62 | 84.92 ± 13.64 | NA |
| 6.46 ± 3.77 | 27.32 ± 8.42 | ||||||||
| 17a | S,S,S | Z | Me | tBu | Bn | Me | 114.22 ± 9.16 | 72.98 ± 14.78 | NA |
| 50.16 ± 11.85 | −23.67 ± 10.83 | ||||||||
As shown in Table 1, N-benzyl and N-n-butyl derivatives 1a,b and 2a,b showed an interesting TRPM8 blockade activity (up to 91 and 60% inhibition of the menthol-induced channel activation at 50 and 5 μM, respectively). In addition, at the indicated concentrations, they are quite selective against TRPV1 (significant blockade was observed only at the high concentration) and especially against TRPA1. Interestingly, Gly analogues 3a,b at the lower concentration (5 μM) decreased TRPM8 activity but were able to nicely block TRPA1 channels. It seems that the incorporation of a polar, H-accepting ester moiety favors TRPA1 recognition, while more hydrophobic residues (Bn, Bu) are preferred at the TRPM8. Interestingly, Ala-derived compounds 4a–c behave as potent and selective TRPV1 antagonists. Thus, a minor modification of compound 3, by the incorporation of a small Me group in 4, gave to a shift in the selectivity, suggesting that this group could occupy a cavity in TRPV1 channels that is not present/accessible in TRPM8 and TRPA1. In general, each distereoisomer of the same compound displayed very similar activities and selectivities. It is interesting to note that the initial ketoester 1 (5 μM) did not show any significant antagonist activity on the channels under study (data not shown).
Modifications at the different parts of molecule 4 also provided us with valuable structural information. Thus, the benzyloxycarbonyl group in 4a,c might be interchanged by a tert-butoxycarbonyl moiety in derivatives 8a,c without apparent loss of TRPV1 antagonist activity, and similar selectivities. However, removal of the urethane moiety and cyclization to the corresponding pyrrolidinone derivatives led to inactive compounds in all studied TRP channels (data not shown), telling us on the importance of a hydrophobic substituent on the Phe nitrogen of 4 and 8.
When the R2 substituent in 4 (Me) is changed by an ethyl group in 9, all diastereoisomers were able to maintain the potent TRPV1 antagonist properties, but an increase in the TRPM8 blocking activity was also detected, specially at the higher concentration. Therefore, Et-derivatives 9 can be considered as dual TRPV1/TRPM8 antagonists.
Concerning the R3 substituent, different results were obtained when the tert-butyl group was replaced by its methyl (10) or benzyl (11) counterparts. While Me derivatives 10a–c were mainly inactive in the three TRP channel assayed, Bn analogues 11a–c lost the TRPV1 antagonist activity, although they showed a significant ability to inhibit the Ca2+ entry through TRPM8 and TRPA1 channels, upon activation with their respective agonists. A similar dual TRPM8/TRPA1 antagonist activity was observed in the case of compounds 14a,c, in which a Phe-OtBu residue was incorporated instead of the Ala-OtBu in 4. The fact that Phe-OtBu and Ala-OBn derived compounds 11 and 14 showed similar activity/selectivity profile could associate the presence of an aromatic ring by this part of the molecule with a preference for TRPM8 and TRPA1 channels.
The role of the R4 benzyl group is not negligible, since compounds with the reverse sequence 15a–b were only able to maintain certain TRPV1 and TRPM8 antagonist activity at high concentrations, and completely loss it for TRPA1 channels. The importance of the phenyl group of this benzyl moiety was corroborated by the lack of activity of the Ile analogue 16b at 5 μM concentration, compared to 4b.
Finally, the decreased TRPV1 antagonist activity of the acetyl derivative 17a, compared to its free NH analogue 4a, suggests a possible direct participation of the NH group either in a saline bridge or in an H-bond formation.
As mentioned previously, no big differences in activity were found among diastereoisomers of the same compound, suggesting that the pocket within the studied TRP channels responsible for the interaction with this family of compounds is quite big, allowing different spatial arrangements to accommodate.
To further validate the TRP-blocking activity for the apparently most potent and selective TRP antagonists, the corresponding dose–response curves were obtained for selected compounds. Table 3 summarizes the IC50 values of N-benzyl derivatives 1a–b for TRPM8 receptors, Gly-analogue 3a for TRPA1, and Ala-derived compounds 4a–c and 8a–c against TRPV1 channels. TRPM8 selective antagonists 1a and 1b show similar micromolar IC50 values, regardless of the configuration at C3. A related value was obtained for the TRPA1 antagonists 3a. Diastereoisomeric Phe–Ala diamino esters 4 and 8 display micromolar or submicromolar IC50 values for TRPV1, quite similar among them, with compound 4b showing the highest potency. Although the configuration does not play a crucial role in the antagonist activity, confirming previous results, the 3R,4S,1′S diastereoisomers seems to give to slightly higher potencies.
| Compd | Configuration 3,4 or 3,4,1′ | Channel | IC50 (μM) |
|---|---|---|---|
| 1a | S,S | TRPM8 | 3.64 ± 0.34 |
| 1b | R,S | TRPM8 | 2.75 ± 0.18 |
| 3a | S,S | TRPA1 | 1.40 ± 0.70 |
| 4a | S,S,S | TRPV1 | 0.62 ± 0.32 |
| 4b | R,S,S | TRPV1 | 0.30 ± 0.33 |
| 4c | S,S,R | TRPV1 | 1.75 ± 0.50 |
| 8a | S,S,S | TRPV1 | 1.13 ± 1.80 |
| 8b | R,S,S | TRPV1 | 0.47 ± 0.80 |
| 8c | S,S,R | TRPV1 | 3.17 ± 0.76 |
In in vivo experiments, compound 4b produced some elevations of PWL in the plantar test, at a 2 mg kg−1 dose ip (see ESI, Fig S1†). Although not statistically significant, it seems that this compound could decrease the thermal hyperalgesia induced in mice by CFA injection. However, no positive signs of activity were observed in the mechanical von Frey test (mechanical hypersensitivity) at this dose. Unfortunately, we could not evaluate the effect at higher doses due to low solubility issues.
Conclusions
In summary, medicinal chemistry efforts initiated from a HTS of synthetic intermediates resulted in the discovery of new selective hits for TRPV1, TRPM8 and TRPA1 blockade, just by incorporating minor changes in the structure of a single β,γ-diaminoester linear scaffold. Compounds were prepared by a two-steps reductive amination process that afforded separable mixtures of diastereoisomers. Starting with Phe-derived β-ketoesters, the SAR demonstrated that the addition of simple 3-NHBn or 3-NHBu substituents resulted in TRPM8 antagonists. Compounds including a second amino acid residue indicated that 3-Gly-OMe-containing derivatives are selective TRPA1 antagonists (3), while the corresponding 3-Ala-OtBu analogues are potent and selective TRPV1 blockers (4). An increase in the hydrophobicity around the R2 substituent enhanced the ability to block the TRPM8 activation, while keeping the TRPV1 antagonists activity, thus resulting in dual TRPV1/TRPM8 blockers. At R3, a tert-butyl ester is clearly preferred for the vanilloid channel while Bn analogues are better for the inhibition of the agonist-activation of both TRPM8 and TRPA1 channels. Dual TRPM8/TRPA1 blockers were also found when the 3-Ala-OtBu moiety was substituted for the more hydrophobic 3-Phe-OBn residue. Concerning the stereochemistry, no strict requirements were observed, with all SSS, RSS and SSR configurations allowed (slightly better results for the RSS diastereoisomers). Although further pharmacological characterization of compounds within this series is limited by their low solubility, and probably poor pharmacokinetics, due in part to the high number of rotable bonds, this work allowed the identification of substituents and amino acid residues that led to selective modulators of the indicated three types of TRP channels. Attaching these particular combinations of substituent on more rigid scaffolds that allowed different 3D-arrangements could serve to discover new potent and selective TRP blockers with improved PK profile. Efforts in this direction are in progress in our labs.Experimental
General methods
All reagents were of commercial quality. Solvents were dried and purified by standard methods. 1H NMR spectra were recorded at 300 or 400 MHz in CDCl3. 13C NMR spectra were registered at 75 MHz. Electrospray mass spectra (positive mode) were also recorded. Analytical TLC was performed on aluminium sheets with a 0.2 mm layer of silica gel F254. Silica gel 60 (230–400 mesh) was used for column chromatography. Silica gel SPE cartridges (Supelco) were also used for compound purification. Analytical HPLC was performed on a Novapak C18 (3.9 × 150 mm, 0.004 mm), Deltapak C18 (3.9 × 150 mm, 0.004 mm) or on a Sunfire C18 (3.9 × 150 mm, 4 μM) column, with a flow rate of 1 mL min−1, using a tuneable UV detector set at 214 nm. Mixtures of MeCN (solvent A) and 0.05% TFA in H2O (solvent B) were used in the mobile phase. The solvent mixtures are specified in each case. Electrospray mass spectra (positive mode) were also recorded. Compounds 1a,b–4a–c were prepared as described.21 β-Ketoesters 5–7, 12 and 13 were prepared by standard procedures, and their analytical and spectroscopic data are coincident with those described.27–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Yield: 19% (syrup). tR = 13.04 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.29–7.18 (m, 5H, Ar), 4.81 (d, 1H, J = 9.2, 4-NH), 3.88 (m, 1H, H-4), 3.63 (s, 3H, OCH3), 3.28 (q, 1H, J = 6.9, H-1′), 2.95 (m, 1H, H-3), 2.80 (d, 2H, J = 7.4, H-5), 2.49 (dd, 1H, J = 15.5, 6.2, H-2), 2.42 (dd, 1H, J = 15.5, 7.2, H-2), 1.68 (bs, 1H, 3-NH), 1.44 (s, 9H, CH3 tBu, Boc), 1.34 (s, 9H, CH3 tBu), 1.24 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.4, 172.2 (CO), 155.5 (NCO), 138.3 (C Ar), 129.2, 128.3, 126.2 (CH Ar), 81.1, 79.9 (C tBu), 57.0 (C1′), 54.9 (C4, C3), 51.6 (OCH3), 39.2 (C5), 38.1 (C2), 28.3, 27.9 (CH3 tBu), 20.20 (C2′). MS: 451.6 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.75; H, 8.44; N, 6.04.
8). Yield: 19% (syrup). tR = 13.04 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.29–7.18 (m, 5H, Ar), 4.81 (d, 1H, J = 9.2, 4-NH), 3.88 (m, 1H, H-4), 3.63 (s, 3H, OCH3), 3.28 (q, 1H, J = 6.9, H-1′), 2.95 (m, 1H, H-3), 2.80 (d, 2H, J = 7.4, H-5), 2.49 (dd, 1H, J = 15.5, 6.2, H-2), 2.42 (dd, 1H, J = 15.5, 7.2, H-2), 1.68 (bs, 1H, 3-NH), 1.44 (s, 9H, CH3 tBu, Boc), 1.34 (s, 9H, CH3 tBu), 1.24 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.4, 172.2 (CO), 155.5 (NCO), 138.3 (C Ar), 129.2, 128.3, 126.2 (CH Ar), 81.1, 79.9 (C tBu), 57.0 (C1′), 54.9 (C4, C3), 51.6 (OCH3), 39.2 (C5), 38.1 (C2), 28.3, 27.9 (CH3 tBu), 20.20 (C2′). MS: 451.6 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.75; H, 8.44; N, 6.04.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Yield: 29% (syrup). tR = 12.42 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.31–7.19 (m, 5H, Ar), 4.98 (d, 1H, J = 8.5, 4-NH), 3.89 (m, 1H, H-4), 3.65 (s, 3H, OCH3), 3.29 (q, 1H, J = 6.8, H-1′), 3.11 (dt, 1H, J = 6.2, 2.8, H-3), 2.89 (dd, 1H, J = 13.6, 6.1, H-5), 2.78 (dd, 1H, J = 13.6, 7.7, H-5), 2.49 (dd, 1H, J = 15.6, 6.3, H-2), 2.39 (dd, 1H, J = 15.6, 6.5, H-2), 1.69 (bs, 1H, 3-NH), 1.47 (s, 9H, CH3 tBu), 1.37 (s, 9H, CH3 tBu), 1.25 (d, 3H, J = 6.8, H-2′). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.2 (CO), 155.6 (NCO), 138.1 (C Ar), 129.3, 128.3, 126.2 (CH Ar), 81.1, 79.0 (C tBu), 55.1 (C1′), 54.9 (C4), 54.5 (C3), 51.6 (OCH3), 38.5 (C5), 37.5 (C2), 28.3, 27.9 (CH3 tBu), 19.8 (C2′). MS: 451.6 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.59; H, 8.61; N, 6.07.
8). Yield: 29% (syrup). tR = 12.42 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.31–7.19 (m, 5H, Ar), 4.98 (d, 1H, J = 8.5, 4-NH), 3.89 (m, 1H, H-4), 3.65 (s, 3H, OCH3), 3.29 (q, 1H, J = 6.8, H-1′), 3.11 (dt, 1H, J = 6.2, 2.8, H-3), 2.89 (dd, 1H, J = 13.6, 6.1, H-5), 2.78 (dd, 1H, J = 13.6, 7.7, H-5), 2.49 (dd, 1H, J = 15.6, 6.3, H-2), 2.39 (dd, 1H, J = 15.6, 6.5, H-2), 1.69 (bs, 1H, 3-NH), 1.47 (s, 9H, CH3 tBu), 1.37 (s, 9H, CH3 tBu), 1.25 (d, 3H, J = 6.8, H-2′). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.2 (CO), 155.6 (NCO), 138.1 (C Ar), 129.3, 128.3, 126.2 (CH Ar), 81.1, 79.0 (C tBu), 55.1 (C1′), 54.9 (C4), 54.5 (C3), 51.6 (OCH3), 38.5 (C5), 37.5 (C2), 28.3, 27.9 (CH3 tBu), 19.8 (C2′). MS: 451.6 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.59; H, 8.61; N, 6.07.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Yield: 20% (syrup). tR = 12.21 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.22–7.09 (m, 5H, Ar), 4.81 (m, 1H, 4-NH), 3.85 (m, 1H, H-4), 3.61 (s, 3H, OCH3), 3.25 (q, 1H, J = 6.9, H-1′), 3.04 (dt, 1H, J = 7.2, 5.7, H-3), 2.86 (dd, 1H, J = 13.6, 5.2, H-5), 2.66 (m, 1H, H-5), 2.50 (dd, 1H, J = 15.2, 5.7, H-2), 2.40 (dd, 1H, J = 15.2, 7.2, H-2), 1.69 (bs, 1H, 3-NH), 1.38 (s, 9H, CH3 tBu), 1.24 (s, 9H, CH3 tBu), 1.14 (d, 3H, J = 6.8, H-2′). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.6 (CO), 155.5 (NCO), 138.2 (C Ar), 129.2, 128.3, 126.3 (CH Ar), 80.9, 79.0 (C tBu), 56.7 (C3), 55.2 (C1′), 54.5 (C4), 51.7 (OCH3), 37.4 (C5), 37.0 (C2), 28.2, 27.9 (CH3 tBu), 19.6 (C2′). MS: 451.6 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.62; H, 8.70; N, 5.98.
8). Yield: 20% (syrup). tR = 12.21 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.22–7.09 (m, 5H, Ar), 4.81 (m, 1H, 4-NH), 3.85 (m, 1H, H-4), 3.61 (s, 3H, OCH3), 3.25 (q, 1H, J = 6.9, H-1′), 3.04 (dt, 1H, J = 7.2, 5.7, H-3), 2.86 (dd, 1H, J = 13.6, 5.2, H-5), 2.66 (m, 1H, H-5), 2.50 (dd, 1H, J = 15.2, 5.7, H-2), 2.40 (dd, 1H, J = 15.2, 7.2, H-2), 1.69 (bs, 1H, 3-NH), 1.38 (s, 9H, CH3 tBu), 1.24 (s, 9H, CH3 tBu), 1.14 (d, 3H, J = 6.8, H-2′). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.6 (CO), 155.5 (NCO), 138.2 (C Ar), 129.2, 128.3, 126.3 (CH Ar), 80.9, 79.0 (C tBu), 56.7 (C3), 55.2 (C1′), 54.5 (C4), 51.7 (OCH3), 37.4 (C5), 37.0 (C2), 28.2, 27.9 (CH3 tBu), 19.6 (C2′). MS: 451.6 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.62; H, 8.70; N, 5.98.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Yield: 16% (syrup). tR = 14.03 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.32–7.19 (m, 10H, Ar), 5.17 (d, 1H, J = 9.3, 4-NH), 5.05, 4.98 (d, 1H, J = 12.1, OCH2 Z), 4.07 (m, 2H, OCH2), 3.97 (m, 1H, H-4), 3.31 (q, 1H, J = 6.8, H-1′), 2.98 (t, 1H, J = 6.1, H-3), 2.84 (d, 2H, J = 7.4, H-5), 2.46 (dd, 1H, J = 15.1, 7.0, H-2), 2.44 (dd, 1H, J = 15.1, 5.5, H-2), 1.69 (bs, 1H, 3-NH), 1.44 (s, 9H, CH3 tBu), 1.25 (d, 3H, J = 7.0, H-2′), 1.20 (t, 3H, J = 7.1, CH3 OEt). 13C NMR (75 MHz, CDCl3) δ: 175.2, 171.5 (CO), 156.1 (NCO), 138.1, 136.0 (C Ar), 129.1, 128.4, 128.3, 127.9, 126.3 (CH Ar), 81.1 (C tBu), 66.4 (OCH2 Z), 60.5 (OCH2), 57.1 (C1′), 55.5 (C4), 54.7 (C3), 39.1 (C5), 38.3 (C2), 27.9 (CH3 tBu), 19.9 (C2′), 14.0 (CH3 OEt). MS: 499.6 [M + 1]+.
8). Yield: 16% (syrup). tR = 14.03 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.32–7.19 (m, 10H, Ar), 5.17 (d, 1H, J = 9.3, 4-NH), 5.05, 4.98 (d, 1H, J = 12.1, OCH2 Z), 4.07 (m, 2H, OCH2), 3.97 (m, 1H, H-4), 3.31 (q, 1H, J = 6.8, H-1′), 2.98 (t, 1H, J = 6.1, H-3), 2.84 (d, 2H, J = 7.4, H-5), 2.46 (dd, 1H, J = 15.1, 7.0, H-2), 2.44 (dd, 1H, J = 15.1, 5.5, H-2), 1.69 (bs, 1H, 3-NH), 1.44 (s, 9H, CH3 tBu), 1.25 (d, 3H, J = 7.0, H-2′), 1.20 (t, 3H, J = 7.1, CH3 OEt). 13C NMR (75 MHz, CDCl3) δ: 175.2, 171.5 (CO), 156.1 (NCO), 138.1, 136.0 (C Ar), 129.1, 128.4, 128.3, 127.9, 126.3 (CH Ar), 81.1 (C tBu), 66.4 (OCH2 Z), 60.5 (OCH2), 57.1 (C1′), 55.5 (C4), 54.7 (C3), 39.1 (C5), 38.3 (C2), 27.9 (CH3 tBu), 19.9 (C2′), 14.0 (CH3 OEt). MS: 499.6 [M + 1]+.Anal. calc. for C28H38N2O6: C, 67.45; H, 7.68; N, 5.62. Found: C, 67.21; H, 7.98; N, 5.49.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Yield: 31% (syrup). tR = 13.22 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.32–7.17 (m, 10H, Ar), 5.36 (d, 1H, J = 8.6, 4-NH), 5.07, 5.00 (d, 1H, J = 12.3, OCH2 Z), 4.01 (m, 2H, OCH2 OEt), 3.95 (m, 1H, H-4), 3.28 (q, 1H, J = 6.9, H-1′), 3.12 (dt, 1H, J = 6.2, 2.9, H-3), 2.93 (dd, 1H, J = 14.0, 5.7, H-5), 2.81 (dd, 1H, J = 14.0, 7.5, H-5), 2.96 (dd, 1H, J = 15.6, 6.0, H-2), 2.81 (dd, 1H, J = 15.6, 6.3, H-2), 1.71 (bs, 1H, 3-NH), 1.45 (s, 9H, CH3 tBu), 1.23 (d, 3H, J = 6.9, H-2′), 1.20 (t, 3H, J = 6.9, CH3 OEt). 13C NMR (75 MHz, CDCl3) δ: 174.9, 171.6 (CO), 156.1 (NCO), 137.8, 136.6 (C Ar), 129.3, 129.2, 128.4, 128.3, 127.9, 127.9, 126.3 (CH Ar), 81.2 (C tBu), 66.4 (OCH2 Z), 60.5 (OCH2), 55.5 (C4), 55.0 (C1′), 54.5 (C3), 39.3 (C5), 37.6 (C2), 27.9 (CH3 tBu), 19.7 (C2′), 14.1 (CH3 OEt). MS: 499.6 [M + 1]+. Anal. calc. for C28H38N2O6: C, 67.45; H, 7.68; N, 5.62. Found: C, 67.33; H, 7.96; N, 5.23.
8). Yield: 31% (syrup). tR = 13.22 min (5 to 100% A in 15 min). 1H NMR (CDCl3) δ: 7.32–7.17 (m, 10H, Ar), 5.36 (d, 1H, J = 8.6, 4-NH), 5.07, 5.00 (d, 1H, J = 12.3, OCH2 Z), 4.01 (m, 2H, OCH2 OEt), 3.95 (m, 1H, H-4), 3.28 (q, 1H, J = 6.9, H-1′), 3.12 (dt, 1H, J = 6.2, 2.9, H-3), 2.93 (dd, 1H, J = 14.0, 5.7, H-5), 2.81 (dd, 1H, J = 14.0, 7.5, H-5), 2.96 (dd, 1H, J = 15.6, 6.0, H-2), 2.81 (dd, 1H, J = 15.6, 6.3, H-2), 1.71 (bs, 1H, 3-NH), 1.45 (s, 9H, CH3 tBu), 1.23 (d, 3H, J = 6.9, H-2′), 1.20 (t, 3H, J = 6.9, CH3 OEt). 13C NMR (75 MHz, CDCl3) δ: 174.9, 171.6 (CO), 156.1 (NCO), 137.8, 136.6 (C Ar), 129.3, 129.2, 128.4, 128.3, 127.9, 127.9, 126.3 (CH Ar), 81.2 (C tBu), 66.4 (OCH2 Z), 60.5 (OCH2), 55.5 (C4), 55.0 (C1′), 54.5 (C3), 39.3 (C5), 37.6 (C2), 27.9 (CH3 tBu), 19.7 (C2′), 14.1 (CH3 OEt). MS: 499.6 [M + 1]+. Anal. calc. for C28H38N2O6: C, 67.45; H, 7.68; N, 5.62. Found: C, 67.33; H, 7.96; N, 5.23.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Yield: 16% (syrup). tR = 13.36 min (5 to 100% A in 15 min). 1H NMR (300 MHz, CDCl3) δ: 7.32–7.19 (m, 10H, Ar), 5.40 (bd, 1H, J = 8.2, 4-NH), 5.03, 4.96 (d, 1H, J = 12.6, OCH2 Z), 4.13 (q, 2H, J = 7.1, OCH2 OEt), 4.00 (m, 1H, H-4), 3.33 (q, 1H, J = 6.9, H-1′), 3.13 (q, 1H, J = 6.5, H-3), 2.93 (dd, 1H, J = 13.8, 5.3, H-5), 2.73 (dd, 1H, J = 13.8, 8.6, H-5), 2.50 (dd, 1H, J = 15.2, 5.9, H-2), 2.42 (dd, 1H, J = 15.2, 7.1 H-2), 1.67 (bs, 1H, 3-NH), 1.42 (s, 9H, CH3 tBu), 1.24 (d, 3H, J = 6.9, H-2′), 1.21 (t, 3H, J = 6.9, CH3 OEt). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.1 (CO), 156.1 (NCO), 138.0, 136.7 (C Ar), 129.2, 128.4, 128.3, 127.8, 127.7, 126.4 (CH Ar), 81.0 (C tBu), 66.3 (OCH2 Z), 60.6 (OCH2 OEt), 56.8 (C3), 55.4 (C4, C1′), 37.3 (C2, C5), 27.9 (CH3 tBu), 19.6 (C2′), 14.1 (CH3 OEt). MS: 499.6 [M + 1]+. Anal. calc. for C28H38N2O6: C, 67.45; H, 7.68; N, 5.62. Found: C, 67.36; H, 7.77; N, 5.35.
8). Yield: 16% (syrup). tR = 13.36 min (5 to 100% A in 15 min). 1H NMR (300 MHz, CDCl3) δ: 7.32–7.19 (m, 10H, Ar), 5.40 (bd, 1H, J = 8.2, 4-NH), 5.03, 4.96 (d, 1H, J = 12.6, OCH2 Z), 4.13 (q, 2H, J = 7.1, OCH2 OEt), 4.00 (m, 1H, H-4), 3.33 (q, 1H, J = 6.9, H-1′), 3.13 (q, 1H, J = 6.5, H-3), 2.93 (dd, 1H, J = 13.8, 5.3, H-5), 2.73 (dd, 1H, J = 13.8, 8.6, H-5), 2.50 (dd, 1H, J = 15.2, 5.9, H-2), 2.42 (dd, 1H, J = 15.2, 7.1 H-2), 1.67 (bs, 1H, 3-NH), 1.42 (s, 9H, CH3 tBu), 1.24 (d, 3H, J = 6.9, H-2′), 1.21 (t, 3H, J = 6.9, CH3 OEt). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.1 (CO), 156.1 (NCO), 138.0, 136.7 (C Ar), 129.2, 128.4, 128.3, 127.8, 127.7, 126.4 (CH Ar), 81.0 (C tBu), 66.3 (OCH2 Z), 60.6 (OCH2 OEt), 56.8 (C3), 55.4 (C4, C1′), 37.3 (C2, C5), 27.9 (CH3 tBu), 19.6 (C2′), 14.1 (CH3 OEt). MS: 499.6 [M + 1]+. Anal. calc. for C28H38N2O6: C, 67.45; H, 7.68; N, 5.62. Found: C, 67.36; H, 7.77; N, 5.35.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield: 16% (syrup). tR = 8.89 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.26–7.13 (m, 10H, Ar), 5.12 (m, 1H, 4-NH), 4.98, 4.89 (d, 1H, J = 12.3, OCH2), 3.92 (m, 1H, H-4), 3.62 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.39 (m, 1H, H-1′), 2.99 (m, 1H, H-3), 2.78 (m, 2H, H-5), 2.45 (m, 2H, H-2), 1.56 (bs, 1H, 3-NH), 1.25 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.8, 172.0 (CO), 156.1 (NCO), 137.8, 136.5 (C Ar), 129.1, 128.4, 127.9, 127.8, 126.4 (CH Ar), 66.5 (OCH2), 56.6 (C4), 55.6 (C3), 55.4 (C1′), 52.0, 51.7 (OCH3), 38.9 (C5), 37.8 (C2), 19.7 (C2′). MS: 443.6 [M + 1]+. Anal. calc. for C24H30N2O6: C, 65.14; H, 6.83; N, 6.33. Found: C, 65.01; H, 6.50; N, 6.39.
2). Yield: 16% (syrup). tR = 8.89 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.26–7.13 (m, 10H, Ar), 5.12 (m, 1H, 4-NH), 4.98, 4.89 (d, 1H, J = 12.3, OCH2), 3.92 (m, 1H, H-4), 3.62 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.39 (m, 1H, H-1′), 2.99 (m, 1H, H-3), 2.78 (m, 2H, H-5), 2.45 (m, 2H, H-2), 1.56 (bs, 1H, 3-NH), 1.25 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.8, 172.0 (CO), 156.1 (NCO), 137.8, 136.5 (C Ar), 129.1, 128.4, 127.9, 127.8, 126.4 (CH Ar), 66.5 (OCH2), 56.6 (C4), 55.6 (C3), 55.4 (C1′), 52.0, 51.7 (OCH3), 38.9 (C5), 37.8 (C2), 19.7 (C2′). MS: 443.6 [M + 1]+. Anal. calc. for C24H30N2O6: C, 65.14; H, 6.83; N, 6.33. Found: C, 65.01; H, 6.50; N, 6.39.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield: 22% (syrup). tR = 7.85 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.28–7.13 (m, 10H, Ar), 5.24 (m, 1H, 4-NH), 4.98, 4.91 (d, 1H, J = 12.6, OCH2), 3.91 (m, 1H, H-4), 3.63 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.40 (m, 1H, H-1′), 3.10 (m, 1H, H-3), 2.80 (m, 2H, H-5), 2.41 (m, 2H, H-2), 1.38 (bs, 1H, 3-NH), 1.22 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.4, 172.1 (CO), 156.2 (NCO), 137.7, 136.5 (C Ar), 129.2, 128.4, 128.3, 127.9, 127.8, 126.4 (CH Ar), 66.5 (OCH2), 55.5 (C4), 54.8 (C3), 54.6 (C1′), 52.0, 51.7 (OCH3), 38.2 (C5), 37.2 (C2), 19.4 (C2′). MS: 443.6 [M + 1]+. Calc. for C24H30N2O6: C, 65.14; H, 6.83; N, 6.33. Found: C, 64.88; H, 7.16; N, 6.00.
2). Yield: 22% (syrup). tR = 7.85 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.28–7.13 (m, 10H, Ar), 5.24 (m, 1H, 4-NH), 4.98, 4.91 (d, 1H, J = 12.6, OCH2), 3.91 (m, 1H, H-4), 3.63 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.40 (m, 1H, H-1′), 3.10 (m, 1H, H-3), 2.80 (m, 2H, H-5), 2.41 (m, 2H, H-2), 1.38 (bs, 1H, 3-NH), 1.22 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.4, 172.1 (CO), 156.2 (NCO), 137.7, 136.5 (C Ar), 129.2, 128.4, 128.3, 127.9, 127.8, 126.4 (CH Ar), 66.5 (OCH2), 55.5 (C4), 54.8 (C3), 54.6 (C1′), 52.0, 51.7 (OCH3), 38.2 (C5), 37.2 (C2), 19.4 (C2′). MS: 443.6 [M + 1]+. Calc. for C24H30N2O6: C, 65.14; H, 6.83; N, 6.33. Found: C, 64.88; H, 7.16; N, 6.00.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield: 23% (syrup). tR = 7.05 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.32–7.18 (m, 10H, Ar), 5.25 (m, 1H, 4-NH), 5.00 (m, 2H, OCH2), 3.99 (m, 1H, H-4), 3.66 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 3.46 (m, 1H, H-1′), 3.14 (m, 1H, H-3), 2.93 (dd, 1H, J = 14.0, 5.2, H-5), 2.75 (m, 1H, H-5), 2.46 (m, 2H, H-2), 1.65 (bs, 1H, 3-NH), 1.25 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.6, 172.4 (CO), 156.1 (NCO), 137.8, 136.5 (C Ar), 129.1, 128.4, 128.3, 127.9, 127.8, 126.5 (CH Ar), 66.5 (OCH2), 56.7 (C3), 55.3 (C4), 54.8 (C1′), 51.9, 51.8 (OCH3), 37.1 (C5), 36.9 (C2), 19.5 (C2′). MS: 443.6 [M + 1]+. Calc. for C24H30N2O6: C, 65.14; H, 6.83; N, 6.33. Found: C, 64.95; H, 6.71; N, 6.05.
2). Yield: 23% (syrup). tR = 7.05 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.32–7.18 (m, 10H, Ar), 5.25 (m, 1H, 4-NH), 5.00 (m, 2H, OCH2), 3.99 (m, 1H, H-4), 3.66 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 3.46 (m, 1H, H-1′), 3.14 (m, 1H, H-3), 2.93 (dd, 1H, J = 14.0, 5.2, H-5), 2.75 (m, 1H, H-5), 2.46 (m, 2H, H-2), 1.65 (bs, 1H, 3-NH), 1.25 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.6, 172.4 (CO), 156.1 (NCO), 137.8, 136.5 (C Ar), 129.1, 128.4, 128.3, 127.9, 127.8, 126.5 (CH Ar), 66.5 (OCH2), 56.7 (C3), 55.3 (C4), 54.8 (C1′), 51.9, 51.8 (OCH3), 37.1 (C5), 36.9 (C2), 19.5 (C2′). MS: 443.6 [M + 1]+. Calc. for C24H30N2O6: C, 65.14; H, 6.83; N, 6.33. Found: C, 64.95; H, 6.71; N, 6.05.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 12% (syrup). tR = 12.05 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.39–7.19 (m, 15H, Ar), 5.19 (m, 1H, 4-NH), 5.17, 5.11 (d, 1H, J = 12.3, OCH2), 5.06, 4.95 (d, 1H, J = 12.4, OCH2), 3.99 (m, 1H, H-4), 3.59 (s, 3H, OCH3), 3.51 (m, 1H, H-1′), 3.08 (m, 1H, H-3), 2.85 (m, 2H, H-5), 2.50 (m, 2H, H-2), 2.05 (bs, 1H, 3-NH), 1.33 (d, 3H, J = 6.8, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.3, 171.9 (CO), 156.1 (NCO), 137.8, 136.5, 135.5 (C Ar), 129.1, 128.6, 128.4, 128.3, 128.2, 127.9, 126.4 (CH Ar), 66.8, 66.5 (OCH2), 56.8 (C1′), 55.5 (C4), 55.4 (C3), 51.7 (OCH3), 38.9 (C5), 37.8 (C2), 19.7 (C2′). MS: 519.6 [M + 1]+. Anal. calc. for C30H34N2O6: C, 69.48; H, 6.61; N, 5.40. Found: C, 69.22; H, 6.62; N, 4.97.
3). Yield: 12% (syrup). tR = 12.05 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.39–7.19 (m, 15H, Ar), 5.19 (m, 1H, 4-NH), 5.17, 5.11 (d, 1H, J = 12.3, OCH2), 5.06, 4.95 (d, 1H, J = 12.4, OCH2), 3.99 (m, 1H, H-4), 3.59 (s, 3H, OCH3), 3.51 (m, 1H, H-1′), 3.08 (m, 1H, H-3), 2.85 (m, 2H, H-5), 2.50 (m, 2H, H-2), 2.05 (bs, 1H, 3-NH), 1.33 (d, 3H, J = 6.8, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.3, 171.9 (CO), 156.1 (NCO), 137.8, 136.5, 135.5 (C Ar), 129.1, 128.6, 128.4, 128.3, 128.2, 127.9, 126.4 (CH Ar), 66.8, 66.5 (OCH2), 56.8 (C1′), 55.5 (C4), 55.4 (C3), 51.7 (OCH3), 38.9 (C5), 37.8 (C2), 19.7 (C2′). MS: 519.6 [M + 1]+. Anal. calc. for C30H34N2O6: C, 69.48; H, 6.61; N, 5.40. Found: C, 69.22; H, 6.62; N, 4.97.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 19% (syrup). tR = 11.60 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.34–7.19 (m, 15H, Ar), 5.20 (m, 1H, 4-NH), 5.15 (m, 2H, OCH2), 5.06, 4.99 (d, 1H, J = 12.3, OCH2), 3.96 (m, 1H, H-4), 3.60 (s, 3H, OCH3), 3.47 (m, 1H, H-1′), 3.15 (m, 1H, H-3), 2.76 (m, 2H, H-5), 2.45 (m, 2H, H-2), 1.67 (bs, 1H, 3-NH), 1.29 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.0 (CO), 156.1 (NCO), 137.7, 136.5, 135.5 (C Ar), 129.2, 128.6, 128.4, 128.3, 128.2, 127.9, 126.4 (CH Ar), 66.7, 66.5 (OCH2Ph), 55.5 (C4), 54.8 (C3), 54.6 (C1′), 51.6 (OCH3), 38.3 (C5), 37.3 (C2), 19.5 (C2′). MS: 519.6 [M + 1]+. Anal. calc. for C30H34N2O6: C, 69.48; H, 6.61; N, 5.40. Found: C, 69.31; H, 6.38; N, 5.48.
3). Yield: 19% (syrup). tR = 11.60 min (20 to 100% A in 20 min). 1H NMR (400 MHz, CDCl3) δ: 7.34–7.19 (m, 15H, Ar), 5.20 (m, 1H, 4-NH), 5.15 (m, 2H, OCH2), 5.06, 4.99 (d, 1H, J = 12.3, OCH2), 3.96 (m, 1H, H-4), 3.60 (s, 3H, OCH3), 3.47 (m, 1H, H-1′), 3.15 (m, 1H, H-3), 2.76 (m, 2H, H-5), 2.45 (m, 2H, H-2), 1.67 (bs, 1H, 3-NH), 1.29 (d, 3H, J = 6.9, H-2′). 13C NMR (75 MHz, CDCl3) δ: 174.9, 172.0 (CO), 156.1 (NCO), 137.7, 136.5, 135.5 (C Ar), 129.2, 128.6, 128.4, 128.3, 128.2, 127.9, 126.4 (CH Ar), 66.7, 66.5 (OCH2Ph), 55.5 (C4), 54.8 (C3), 54.6 (C1′), 51.6 (OCH3), 38.3 (C5), 37.3 (C2), 19.5 (C2′). MS: 519.6 [M + 1]+. Anal. calc. for C30H34N2O6: C, 69.48; H, 6.61; N, 5.40. Found: C, 69.31; H, 6.38; N, 5.48.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 29% (syrup). tR = 10.19 min (20 to 100% A in 20 min). Diastereosiomeric mixture c
3). Yield: 29% (syrup). tR = 10.19 min (20 to 100% A in 20 min). Diastereosiomeric mixture c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d = 1
d = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1H NMR (300 MHz, CDCl3) δ: 7.35–7.15 (m, 15H, Ar), 5.24 (d, 1H, J = 8.0, 4-NH), 5.15, 5.09 (d, 1H, J = 12.3, OCH2), 4.98 (m, 2H, OCH2), 3.98 (m, 1H, H-4), 3.64, 3.62 (s, 3H, OCH3), 3.51 (m, 1H, H-3′), 3.16, 3.10 (q, 1H, J = 6.0, H-1′), 2.92 (m, 1H, H-5), 2.72 (m, 1H, H-5), 2.48 (m, 2H, H-2), 1.60 (bs, 1H, 3-NH), 1.28, 1.27 (d, 3H, J = 7.0, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.7, 175.4 (COO), 172.6, 172.4 (COO), 156.3, 156.1 (NCO), 138.1, 138.0, 136.8, 136.7, 135.85, 135.8 (C Ar), 128.7, 128.6, 128.4, 128.3, 128.1, 127.9, 126.6 (CH Ar), 66.8, 66.6 (OCH2), 57.0, 56.7 (C3), 55.0 (C4), 55.1, 55.0 (C1′), 51.1, 51.0 (OCH3), 37.4, 37.2, 37.1 (C5, C2), 19.9, 19.6 (C2′). MS: 519.5 [M + 1]+.
1. 1H NMR (300 MHz, CDCl3) δ: 7.35–7.15 (m, 15H, Ar), 5.24 (d, 1H, J = 8.0, 4-NH), 5.15, 5.09 (d, 1H, J = 12.3, OCH2), 4.98 (m, 2H, OCH2), 3.98 (m, 1H, H-4), 3.64, 3.62 (s, 3H, OCH3), 3.51 (m, 1H, H-3′), 3.16, 3.10 (q, 1H, J = 6.0, H-1′), 2.92 (m, 1H, H-5), 2.72 (m, 1H, H-5), 2.48 (m, 2H, H-2), 1.60 (bs, 1H, 3-NH), 1.28, 1.27 (d, 3H, J = 7.0, H-2′). 13C NMR (75 MHz, CDCl3) δ: 175.7, 175.4 (COO), 172.6, 172.4 (COO), 156.3, 156.1 (NCO), 138.1, 138.0, 136.8, 136.7, 135.85, 135.8 (C Ar), 128.7, 128.6, 128.4, 128.3, 128.1, 127.9, 126.6 (CH Ar), 66.8, 66.6 (OCH2), 57.0, 56.7 (C3), 55.0 (C4), 55.1, 55.0 (C1′), 51.1, 51.0 (OCH3), 37.4, 37.2, 37.1 (C5, C2), 19.9, 19.6 (C2′). MS: 519.5 [M + 1]+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 15% (syrup). tR = 15.43 min (20 to 100% A in 20 min). 1H NMR (CDCl3) δ: 7.33–6.96 (m, 15H, Ar), 5.03, 4.95 (d, 1H, J = 12.4 Hz, OCH2), 4.90 (bd, 1H, J = 10.0 Hz, 4-NH), 3.81 (m, 1H, H-4), 3.61 (s, 3H, OCH3), 3.42 (m, 1H, H-1′), 2.95 (m, 2H, H-3, H-5), 2.71 (dd, 1H, J = 8.6, 13.3 Hz, H-5), 2.46 (m, 4H, H-2, H-2′), 1.38 (s, 9H, CH3 tBu). 13C NMR (75 MHz, CDCl3) δ: 174.5, 171.7 (COO), 156.0 (NCO), 138.0, 136.6 (C Ar), 129.6, 129.1, 128.4, 128.3, 128.2, 127.9, 127.8, 126.6, 126.2 (CH Ar), 81.5 (C tBu), 66.4 (OCH2), 64.0 (C1′), 55.7 (C4), 55.3 (C3), 51.6 (OCH3), 40.7 (C5), 38.9 (C2′), 38.3 (C2), 27.9 (CH3 tBu). MS: 561.3 [M + 1]+, 583.3 [M + 23]+. Anal. calc. for C33H40N2O6: C, 70.69; H, 7.19; N, 5.00. Found: C, 70.34; H, 7.02; N, 5.21.
4). Yield: 15% (syrup). tR = 15.43 min (20 to 100% A in 20 min). 1H NMR (CDCl3) δ: 7.33–6.96 (m, 15H, Ar), 5.03, 4.95 (d, 1H, J = 12.4 Hz, OCH2), 4.90 (bd, 1H, J = 10.0 Hz, 4-NH), 3.81 (m, 1H, H-4), 3.61 (s, 3H, OCH3), 3.42 (m, 1H, H-1′), 2.95 (m, 2H, H-3, H-5), 2.71 (dd, 1H, J = 8.6, 13.3 Hz, H-5), 2.46 (m, 4H, H-2, H-2′), 1.38 (s, 9H, CH3 tBu). 13C NMR (75 MHz, CDCl3) δ: 174.5, 171.7 (COO), 156.0 (NCO), 138.0, 136.6 (C Ar), 129.6, 129.1, 128.4, 128.3, 128.2, 127.9, 127.8, 126.6, 126.2 (CH Ar), 81.5 (C tBu), 66.4 (OCH2), 64.0 (C1′), 55.7 (C4), 55.3 (C3), 51.6 (OCH3), 40.7 (C5), 38.9 (C2′), 38.3 (C2), 27.9 (CH3 tBu). MS: 561.3 [M + 1]+, 583.3 [M + 23]+. Anal. calc. for C33H40N2O6: C, 70.69; H, 7.19; N, 5.00. Found: C, 70.34; H, 7.02; N, 5.21.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 28% (syrup). tR = 13.48 min (20 to 100% A in 20 min). 1H NMR (CDCl3) δ: 7.33–7.15 (m, 15H, Ar), 5.23 (bd, 1H, J = 7.2 Hz, 4-NH), 5.06, 5.00 (d, 1H, J = 12.3 Hz, OCH2), 3.91 (m, 1H, H-4), 3.56 (s, 3H, OCH3), 3.44 (m, 1H, H-1′), 3.07 (m, 1H, H-3), 2.85 (m, 4H, H-5, H-2′), 2.31 (m, 2H, H-2), 1.33 (s, 9H, CH3 tBu). 13C NMR (75 MHz, CDCl3) δ: 173.6, 171.9 (COO), 156.0 (NCO), 137.7, 137.1, 136.6 (C Ar), 129.4, 129.3, 128.5, 128.4, 128.3, 128.0, 127.9, 126.7, 126.4 (CH Ar), 81.6 (C tBu), 66.5 (OCH2), 61.1 (C1′), 55.3 (C4), 54.5 (C3), 51.6 (OCH3), 40.3, 38.4 (C5, C2′), 36.9 (C2), 27.9 (CH3 tBu). MS: 561.3 [M + 1]+, 583.3 [M + 23]+. Anal. calc. for C33H40N2O6: C, 70.69; H, 7.19; N, 5.00. Found: C, 70.85; H, 7.15; N, 4.86.
4). Yield: 28% (syrup). tR = 13.48 min (20 to 100% A in 20 min). 1H NMR (CDCl3) δ: 7.33–7.15 (m, 15H, Ar), 5.23 (bd, 1H, J = 7.2 Hz, 4-NH), 5.06, 5.00 (d, 1H, J = 12.3 Hz, OCH2), 3.91 (m, 1H, H-4), 3.56 (s, 3H, OCH3), 3.44 (m, 1H, H-1′), 3.07 (m, 1H, H-3), 2.85 (m, 4H, H-5, H-2′), 2.31 (m, 2H, H-2), 1.33 (s, 9H, CH3 tBu). 13C NMR (75 MHz, CDCl3) δ: 173.6, 171.9 (COO), 156.0 (NCO), 137.7, 137.1, 136.6 (C Ar), 129.4, 129.3, 128.5, 128.4, 128.3, 128.0, 127.9, 126.7, 126.4 (CH Ar), 81.6 (C tBu), 66.5 (OCH2), 61.1 (C1′), 55.3 (C4), 54.5 (C3), 51.6 (OCH3), 40.3, 38.4 (C5, C2′), 36.9 (C2), 27.9 (CH3 tBu). MS: 561.3 [M + 1]+, 583.3 [M + 23]+. Anal. calc. for C33H40N2O6: C, 70.69; H, 7.19; N, 5.00. Found: C, 70.85; H, 7.15; N, 4.86.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 21% (syrup). tR = 13.48 min (20 to 100% A in 20 min). Diastereoisomeric mixture c
4). Yield: 21% (syrup). tR = 13.48 min (20 to 100% A in 20 min). Diastereoisomeric mixture c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d = 2.5
d = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1H NMR (300 MHz, CDCl3) δ: 7.34–7.05 (m, 15H, Ar), 5.33 (bd, 1H, J = 10.6 Hz, 4-NH isomer d), 5.00 (s, 2H, OCH2 d), 4.93 (s, 2H, OCH2 isomer c), 4.59 (bd, 1H, J = 8.2 Hz, 4-NH isomer c), 3.97 (m, 1H, H-4 d), 3.83 (m, 1H, H-4 c), 3.63 (s, 3H, OCH3 c), 3.56 (s, 3H, OCH3 d), 3.47 (dd, 1H, J = 8.0, 6.0, H-2′ d), 3.47 (dd, 1H, J = 8.5, 5.7, H-2′ c), 3.08 (m, 1H, H-3 d), 2.97 (m, 1H, H-3 d), 2.96–2.64 (m, 2H, H-5, H-3′), 2.46 (m, 4H, H-2 c), 2.25 (m, 4H, H-2 d), 1.37 (s, 9H, CH3 tBu c), 1.36 (s, 9H, CH3 tBu d). MS: 561.2 [M + 1]+.
1. 1H NMR (300 MHz, CDCl3) δ: 7.34–7.05 (m, 15H, Ar), 5.33 (bd, 1H, J = 10.6 Hz, 4-NH isomer d), 5.00 (s, 2H, OCH2 d), 4.93 (s, 2H, OCH2 isomer c), 4.59 (bd, 1H, J = 8.2 Hz, 4-NH isomer c), 3.97 (m, 1H, H-4 d), 3.83 (m, 1H, H-4 c), 3.63 (s, 3H, OCH3 c), 3.56 (s, 3H, OCH3 d), 3.47 (dd, 1H, J = 8.0, 6.0, H-2′ d), 3.47 (dd, 1H, J = 8.5, 5.7, H-2′ c), 3.08 (m, 1H, H-3 d), 2.97 (m, 1H, H-3 d), 2.96–2.64 (m, 2H, H-5, H-3′), 2.46 (m, 4H, H-2 c), 2.25 (m, 4H, H-2 d), 1.37 (s, 9H, CH3 tBu c), 1.36 (s, 9H, CH3 tBu d). MS: 561.2 [M + 1]+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 6% (syrup). tR = 13.97 min (20 to 100% A in 30 min). 1H NMR (CDCl3) δ: 7.28–7.10 (m, 10H, Ar), 4.99 (m, 2H, OCH2), 4.84 (m, 1H, 4-NH), 3.57 (m, 4H, H-1′, OCH3), 3.32 (m, 1H, H-4), 2.80 (m, 2H, H-3, H-2′), 2.67 (dd, 1H, J = 13.4, 8.0, H-2′), 2.34 (m, 2H, H-2), 1.52 (bs, 1H, 3-NH), 1.30 (s, 9H, CH3 tBu), 0.85 (d, 3H, J = 6.7, H-5). 13C NMR (75 MHz, CDCl3) δ: 174.3, 172.1 (CO), 155.9 (NCO), 137.7, 136.6 (C Ar), 129.5, 128.5, 128.2, 128.0, 126.5 (CH Ar), 81.5 (C tBu), 66.5 (OCH2), 63.7 (C4), 57.6 (C3), 51.7 (OCH3), 49.9 (C1′), 40.5 (C2′), 37.9 (C2), 27.9 (CH3 tBu), 18.5 (C5). MS: 485.6 [M + 1]+. Anal. calc. for C27H36N2O6: C, 66.92; H, 7.49; N, 5.78. Found: C, 66.73; H, 7.12; N, 5.32.
3). Yield: 6% (syrup). tR = 13.97 min (20 to 100% A in 30 min). 1H NMR (CDCl3) δ: 7.28–7.10 (m, 10H, Ar), 4.99 (m, 2H, OCH2), 4.84 (m, 1H, 4-NH), 3.57 (m, 4H, H-1′, OCH3), 3.32 (m, 1H, H-4), 2.80 (m, 2H, H-3, H-2′), 2.67 (dd, 1H, J = 13.4, 8.0, H-2′), 2.34 (m, 2H, H-2), 1.52 (bs, 1H, 3-NH), 1.30 (s, 9H, CH3 tBu), 0.85 (d, 3H, J = 6.7, H-5). 13C NMR (75 MHz, CDCl3) δ: 174.3, 172.1 (CO), 155.9 (NCO), 137.7, 136.6 (C Ar), 129.5, 128.5, 128.2, 128.0, 126.5 (CH Ar), 81.5 (C tBu), 66.5 (OCH2), 63.7 (C4), 57.6 (C3), 51.7 (OCH3), 49.9 (C1′), 40.5 (C2′), 37.9 (C2), 27.9 (CH3 tBu), 18.5 (C5). MS: 485.6 [M + 1]+. Anal. calc. for C27H36N2O6: C, 66.92; H, 7.49; N, 5.78. Found: C, 66.73; H, 7.12; N, 5.32.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 12% (syrup). tR = 13.37 min (20 to 100% A in 30 min). 1H NMR (CDCl3) δ: 7.37–7.18 (m, 10H, Ar), 5.29 (bd, 1H, J = 7.9, 4-NH), 5.11 (m, 2H, OCH2), 3.73 (m, 1H, H-4), 3.60 (s, 3H, OCH3), 3.43 (m, 1H, H-1′), 2.98 (m, 1H, H-3), 2.85 (m, 2H, H-2′), 2.36 (dd, 1H, J = 15.6, 6.3, H-2), 2.27 (dd, 1H, J = 15.6, 6.1, H-2), 1.45 (s, 9H, CH3 tBu), 1.17 (d, 3H, J = 6.7, H-5). 13C NMR (75 MHz, CDCl3) δ: 173.8, 172.5 (COO), 156.2 (NCO), 137.4, 136.8 (C Ar), 129.5, 128.6, 128.4, 128.1, 126.7 (CH Ar), 81.7 (C tBu), 66.7 (OCH2), 61.0 (C1′), 57.1 (C3), 51.8 (OCH3), 49.9 (C4), 40.3 (C2′), 36.7 (C2), 28.1 (CH3 tBu), 18.3 (C5). MS: 485.2 [M + 1]+. Anal. calc. for C27H36N2O6: C, 66.92; H, 7.49; N, 5.78. Found: C, 66.58; H, 7.83; N, 5.40.
3). Yield: 12% (syrup). tR = 13.37 min (20 to 100% A in 30 min). 1H NMR (CDCl3) δ: 7.37–7.18 (m, 10H, Ar), 5.29 (bd, 1H, J = 7.9, 4-NH), 5.11 (m, 2H, OCH2), 3.73 (m, 1H, H-4), 3.60 (s, 3H, OCH3), 3.43 (m, 1H, H-1′), 2.98 (m, 1H, H-3), 2.85 (m, 2H, H-2′), 2.36 (dd, 1H, J = 15.6, 6.3, H-2), 2.27 (dd, 1H, J = 15.6, 6.1, H-2), 1.45 (s, 9H, CH3 tBu), 1.17 (d, 3H, J = 6.7, H-5). 13C NMR (75 MHz, CDCl3) δ: 173.8, 172.5 (COO), 156.2 (NCO), 137.4, 136.8 (C Ar), 129.5, 128.6, 128.4, 128.1, 126.7 (CH Ar), 81.7 (C tBu), 66.7 (OCH2), 61.0 (C1′), 57.1 (C3), 51.8 (OCH3), 49.9 (C4), 40.3 (C2′), 36.7 (C2), 28.1 (CH3 tBu), 18.3 (C5). MS: 485.2 [M + 1]+. Anal. calc. for C27H36N2O6: C, 66.92; H, 7.49; N, 5.78. Found: C, 66.58; H, 7.83; N, 5.40.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 15b,c. (CH2Cl2
1 mixture of 15b,c. (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ether
ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 42% (syrup). tR = 13.24 min (20 to 100% A in 30 min). 1H NMR (CDCl3) δ: 7.37–7.18 (m, 10H, Ar), 5.77 (bd, 1H, J = 8.1, 4-NH), 5.11 (m, 2H, OCH2), 3.66 (m, 1H, H-4), 3.60 (s, 3H, OCH3), 3.48 (m, 1H, H-1′), 2.98 (m, 2H, H-3, H-2′), 2.75 (dd, 1H, J = 13.5, 8.3, H-2′), 2.19 (m, 2H, H-2), 1.46 (s, 9H, CH3 tBu), 1.05 (d, 3H, J = 6.7, H-5). 13C NMR (75 MHz, CDCl3) δ: 174.1, 172.2 (COO), 156.0 (NCO), 137.4, 137.0 (C Ar), 129.4, 128.6, 128.5, 128.1, 126.9 (CH Ar), 81.7 (C tBu), 66.5 (OCH2), 61.6 (C1′), 57.5 (C3), 51.9 (OCH3), 49.6 (C4), 40.0 (C2′), 37.3 (C2), 28.1 (CH3 tBu), 15.5 (C5). MS: 485.4 [M + 1]+.
3). Yield: 42% (syrup). tR = 13.24 min (20 to 100% A in 30 min). 1H NMR (CDCl3) δ: 7.37–7.18 (m, 10H, Ar), 5.77 (bd, 1H, J = 8.1, 4-NH), 5.11 (m, 2H, OCH2), 3.66 (m, 1H, H-4), 3.60 (s, 3H, OCH3), 3.48 (m, 1H, H-1′), 2.98 (m, 2H, H-3, H-2′), 2.75 (dd, 1H, J = 13.5, 8.3, H-2′), 2.19 (m, 2H, H-2), 1.46 (s, 9H, CH3 tBu), 1.05 (d, 3H, J = 6.7, H-5). 13C NMR (75 MHz, CDCl3) δ: 174.1, 172.2 (COO), 156.0 (NCO), 137.4, 137.0 (C Ar), 129.4, 128.6, 128.5, 128.1, 126.9 (CH Ar), 81.7 (C tBu), 66.5 (OCH2), 61.6 (C1′), 57.5 (C3), 51.9 (OCH3), 49.6 (C4), 40.0 (C2′), 37.3 (C2), 28.1 (CH3 tBu), 15.5 (C5). MS: 485.4 [M + 1]+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Yield: 13% (syrup). tR = 4.78 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ: 7.36 (m, 5H, Ar), 5.20 (bd, 1H, J = 7.2 Hz, 4-NH), 5.12, 5.06 (d, 1H, J = 12.1 Hz, OCH2), 3.66 (s, 3H, OCH3), 3.25 (m, 1H, H-3, H-4, H-1′), 2.45 (m, 2H, H-2), 1.51 (m, 2H, H-5, H-6), 1.45 (s, 9H, CH3 tBu), 1.19 (d, 3H, J = 6.9, H-2′), 1.08 (m, 1H, H-6), 0.96 (d, 3H, J = 6.7, 5-CH3), 0.86 (t, 3H, J = 7.3, H-7). 13C NMR (CDCl3) δ: 175.5, 172.3 (COO), 156.8 (NCO), 136.9 (C Ar), 128.6, 128.2, 127.9 (CH Ar), 81.3 (C tBu), 66.8 (OCH2), 59.3 (C4), 57.2 (C-1′), 53.3 (C3), 51.8 (OCH3), 38.8 (C2), 37.1 (C5), 28.1 (CH3 tBu), 26.7 (C6), 20.0 (C2′), 15.9 (5-CH3), 11.2 (C7). MS: 451.4 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.91; H, 8.58; N, 5.89.
7). Yield: 13% (syrup). tR = 4.78 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ: 7.36 (m, 5H, Ar), 5.20 (bd, 1H, J = 7.2 Hz, 4-NH), 5.12, 5.06 (d, 1H, J = 12.1 Hz, OCH2), 3.66 (s, 3H, OCH3), 3.25 (m, 1H, H-3, H-4, H-1′), 2.45 (m, 2H, H-2), 1.51 (m, 2H, H-5, H-6), 1.45 (s, 9H, CH3 tBu), 1.19 (d, 3H, J = 6.9, H-2′), 1.08 (m, 1H, H-6), 0.96 (d, 3H, J = 6.7, 5-CH3), 0.86 (t, 3H, J = 7.3, H-7). 13C NMR (CDCl3) δ: 175.5, 172.3 (COO), 156.8 (NCO), 136.9 (C Ar), 128.6, 128.2, 127.9 (CH Ar), 81.3 (C tBu), 66.8 (OCH2), 59.3 (C4), 57.2 (C-1′), 53.3 (C3), 51.8 (OCH3), 38.8 (C2), 37.1 (C5), 28.1 (CH3 tBu), 26.7 (C6), 20.0 (C2′), 15.9 (5-CH3), 11.2 (C7). MS: 451.4 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.91; H, 8.58; N, 5.89.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Yield: 21% (syrup). tR = 4.36 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ: 7.34 (m, 5H, Ar), 5.27 (bs, 1H, 4-NH), 5.11 (s, 2H, OCH2), 3.66 (s, 3H, OCH3), 3.53 (m, 1H, H-4), 3.24 (m, 2H, H-3, H-1′), 2.43 (m, 2H, H-2), 1.56 (m, 1H, H-5), 1.45 (s, 9H, CH3 tBu), 1.24 (m, 2H, H-6), 1.20 (d, 3H, J = 6.9, H-2′), 0.91 (t, 3H, J = 7.2, H-7), 0.88 (d, 3H, J = 6.5, 5-CH3). 13C NMR (CDCl3) δ: 175.2, 172.4 (COO), 157.1 (NCO), 136.9 (C Ar), 128.6, 128.1, 128.0 (CH Ar), 81.3 (C tBu), 66.7 (OCH2), 58.0 (C4), 54.9 (C-1′), 53.2 (C3), 51.8 (OCH3), 37.7 (C2), 36.4 (C5), 28.0 (CH3 tBu), 26.3 (C6), 20.0 (C2′), 15.1 (5-CH3), 11.0 (C7). MS: 451.1 [M + 1]. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.71; H, 8.20; N, 5.95.
7). Yield: 21% (syrup). tR = 4.36 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ: 7.34 (m, 5H, Ar), 5.27 (bs, 1H, 4-NH), 5.11 (s, 2H, OCH2), 3.66 (s, 3H, OCH3), 3.53 (m, 1H, H-4), 3.24 (m, 2H, H-3, H-1′), 2.43 (m, 2H, H-2), 1.56 (m, 1H, H-5), 1.45 (s, 9H, CH3 tBu), 1.24 (m, 2H, H-6), 1.20 (d, 3H, J = 6.9, H-2′), 0.91 (t, 3H, J = 7.2, H-7), 0.88 (d, 3H, J = 6.5, 5-CH3). 13C NMR (CDCl3) δ: 175.2, 172.4 (COO), 157.1 (NCO), 136.9 (C Ar), 128.6, 128.1, 128.0 (CH Ar), 81.3 (C tBu), 66.7 (OCH2), 58.0 (C4), 54.9 (C-1′), 53.2 (C3), 51.8 (OCH3), 37.7 (C2), 36.4 (C5), 28.0 (CH3 tBu), 26.3 (C6), 20.0 (C2′), 15.1 (5-CH3), 11.0 (C7). MS: 451.1 [M + 1]. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.71; H, 8.20; N, 5.95.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (EtOAc
2 (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Yield: 16% (syrup), mixture of D2 and D3 in 1
7). Yield: 16% (syrup), mixture of D2 and D3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. tR = 3.82 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ D3: 7.34 (m, 5H, Ar), 5.09, 5.05 (d, 1H, J = 12.3 Hz, OCH2), 4.67 (d, 1H, J = 10.5, 4-NH), 3.64 (m, 1H, H-4), 3.58 (s, 3H, OCH3), 3.20 (m, 1H, H-1′), 3.05 (m, 2H, H-3), 2.41 (m, 2H, H-2), 1.82 (m, 1H, H-5), 1.54 (m, 1H, H-6), 1.44 (s, 9H, CH3 tBu), 1.37 (m, 1H, H-6), 1.18 (d, 3H, J = 6.9, H-2′), 0.90 (t, 3H, J = 7.2, H-7), 0.84 (d, 3H, J = 6.8, 5-CH3). 13C NMR (CDCl3) δ: 175.1, 173.7 (COO), 157.0 (NCO), 136.9 (C Ar), 128.6, 128.3, 128.1 (CH Ar), 82.5 (C tBu), 66.8 (OCH2), 57.3 (C4), 55.2 (C-1′), 53.9 (C3), 51.7 (OCH3), 37.1 (C2), 34.9 (C5), 28.0 (CH3 tBu), 27.1 (C6), 19.7 (C2′), 15.7 (5-CH3), 11.5 (C7). MS: 451.5 [M + 1]+.
2 ratio. tR = 3.82 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ D3: 7.34 (m, 5H, Ar), 5.09, 5.05 (d, 1H, J = 12.3 Hz, OCH2), 4.67 (d, 1H, J = 10.5, 4-NH), 3.64 (m, 1H, H-4), 3.58 (s, 3H, OCH3), 3.20 (m, 1H, H-1′), 3.05 (m, 2H, H-3), 2.41 (m, 2H, H-2), 1.82 (m, 1H, H-5), 1.54 (m, 1H, H-6), 1.44 (s, 9H, CH3 tBu), 1.37 (m, 1H, H-6), 1.18 (d, 3H, J = 6.9, H-2′), 0.90 (t, 3H, J = 7.2, H-7), 0.84 (d, 3H, J = 6.8, 5-CH3). 13C NMR (CDCl3) δ: 175.1, 173.7 (COO), 157.0 (NCO), 136.9 (C Ar), 128.6, 128.3, 128.1 (CH Ar), 82.5 (C tBu), 66.8 (OCH2), 57.3 (C4), 55.2 (C-1′), 53.9 (C3), 51.7 (OCH3), 37.1 (C2), 34.9 (C5), 28.0 (CH3 tBu), 27.1 (C6), 19.7 (C2′), 15.7 (5-CH3), 11.5 (C7). MS: 451.5 [M + 1]+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, 1
hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Yield: 6% (syrup). tR = 3.64 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ: 7.35 (m, 5H, Ar), 5.08 (s, 2H, OCH2), 5.20 (d, 1H, J = 10.3 Hz, 4-NH), 3.61 (m, 4H, H-4, OCH3), 3.30 (m, 1H, H-1′), 3.19 (m, 1H, H-3), 2.42 (dd, 1H, J = 15.3, 5.5, H-2), 2.32 (dd, 1H, J = 15.3, 6.3, H-2), 1.56 (m, 2H, H-5, H-6), 1.45 (s, 9H, CH3 tBu), 1.21 (d, 3H, J = 6.9, H-2′), 1.00 (m, 1H, H-6), 0.93 (d, 3H, J = 6.5, 5-CH3), 0.90 (t, 3H, J = 7.2, H-7). 13C NMR (CDCl3) δ: 174.9, 173.3 (COO), 157.0 (NCO), 136.8 (C Ar), 128.6, 128.2, 128.1 (CH Ar), 80.9 (C tBu), 67.0 (OCH2), 59.1 (C4), 54.8 (C-1′), 53.3 (C3), 51.7 (OCH3), 36.4 (C2), 35.9 (C5), 28.1 (CH3 tBu), 24.4 (C6), 19.7 (C2′), 16.5 (5-CH3), 11.5 (C7). MS: 451.1 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.86; H, 8.09; N, 6.05.
7). Yield: 6% (syrup). tR = 3.64 min (15 to 95% A in 5 min). 1H NMR (CDCl3) δ: 7.35 (m, 5H, Ar), 5.08 (s, 2H, OCH2), 5.20 (d, 1H, J = 10.3 Hz, 4-NH), 3.61 (m, 4H, H-4, OCH3), 3.30 (m, 1H, H-1′), 3.19 (m, 1H, H-3), 2.42 (dd, 1H, J = 15.3, 5.5, H-2), 2.32 (dd, 1H, J = 15.3, 6.3, H-2), 1.56 (m, 2H, H-5, H-6), 1.45 (s, 9H, CH3 tBu), 1.21 (d, 3H, J = 6.9, H-2′), 1.00 (m, 1H, H-6), 0.93 (d, 3H, J = 6.5, 5-CH3), 0.90 (t, 3H, J = 7.2, H-7). 13C NMR (CDCl3) δ: 174.9, 173.3 (COO), 157.0 (NCO), 136.8 (C Ar), 128.6, 128.2, 128.1 (CH Ar), 80.9 (C tBu), 67.0 (OCH2), 59.1 (C4), 54.8 (C-1′), 53.3 (C3), 51.7 (OCH3), 36.4 (C2), 35.9 (C5), 28.1 (CH3 tBu), 24.4 (C6), 19.7 (C2′), 16.5 (5-CH3), 11.5 (C7). MS: 451.1 [M + 1]+. Anal. calc. for C24H38N2O6: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.86; H, 8.09; N, 6.05.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. The title compound, 48 mg (86%) was obtained as a syrup. tR = 8.52 min (15 to 95% A in 10 min). 1H NMR (300 MHz, CDCl3) δ: 7.30–7.00 (m, 10H, Ar), 5.81 (d, 1H, J = 8.5, 4-NH), 4.90 (m, 2H, OCH2), 4.59 (m, 1H, H-4), 4.00 (m, 1H, H-2′), 3.65 (m, 1H, H-3), 3.59 (s, 3H, OCH3), 3.12 (m, 1H, H-5), 2.91 (m, 1H, H-5), 2.68 (m, 2H, H-2), 2.15 (s, 3H, CH3 Ac), 1.45 (s, 9H, CH3 tBu), 1.26 (d, 3H, J = 7.1, H-3′). MS: 527.6 [M + 1]+. Anal. calc. for C29H38N2O7: C, 66.14; H, 7.27; N, 5.32. Found: C, 65.87; H, 6.95; N, 5.01.
1) as eluent. The title compound, 48 mg (86%) was obtained as a syrup. tR = 8.52 min (15 to 95% A in 10 min). 1H NMR (300 MHz, CDCl3) δ: 7.30–7.00 (m, 10H, Ar), 5.81 (d, 1H, J = 8.5, 4-NH), 4.90 (m, 2H, OCH2), 4.59 (m, 1H, H-4), 4.00 (m, 1H, H-2′), 3.65 (m, 1H, H-3), 3.59 (s, 3H, OCH3), 3.12 (m, 1H, H-5), 2.91 (m, 1H, H-5), 2.68 (m, 2H, H-2), 2.15 (s, 3H, CH3 Ac), 1.45 (s, 9H, CH3 tBu), 1.26 (d, 3H, J = 7.1, H-3′). MS: 527.6 [M + 1]+. Anal. calc. for C29H38N2O7: C, 66.14; H, 7.27; N, 5.32. Found: C, 65.87; H, 6.95; N, 5.01.Calcium microfluorography
For fluorescence assays, cells expressing TRP channels (TRPV1-SH-SY5Y, TRPM8-HEK and TRPA1-IMR90) were seeded in 96-well plates (Corning Incorporated, Corning, NY) at a cell density of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells 2 days before treatment. The day of treatment the medium was replaced with 100 μL of the dye loading solution Fluo-4 NW supplemented with probenecid 2.5 mM. Then the compounds dissolved in DMSO were added at the desired concentrations and the plate(s) were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 60 minutes.
000 cells 2 days before treatment. The day of treatment the medium was replaced with 100 μL of the dye loading solution Fluo-4 NW supplemented with probenecid 2.5 mM. Then the compounds dissolved in DMSO were added at the desired concentrations and the plate(s) were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 60 minutes.
The fluorescence was measured using instrument settings appropriate for excitation at 485 nm and emission at 535 nm (POLARstar Omega BMG LAB tech). A baseline recording of 7 cycles was recorded prior to stimulation with the agonist (10 μM capsaicin for TRPV1, 100 μM menthol for TRPM8, and 100 μM AITC for TRPA1). The corresponding antagonist (10 μM ruthenium red for TRPV1 and TRPA1, 100 μM AMTB for TRPM8) was added for the blockade. The changes in fluorescence intensity were recorded during 15 cycles more. DMSO, at the higher concentration used in the experiment, was added to the control wells.
The degree of blockage (%) of TRP channel activity was calculated by:
where F0 is the fluorescence after the addition of agonist in the presence of the compound, F1 is the fluorescence before the addition of agonist in the presence of the compound, Fc0 is the fluorescence after the addition of agonist in the absence of the compound, Fc1 is the fluorescence before the addition of agonist in the absence of the compound.
The Z factor was calculated using the following equation:
where meanmax is the mean of the maximum fluorescence in the presence of agonist, meanmin is the mean of the maximum fluorescence in the presence of agonist and antagonist.
Acknowledgements
This work was supported by the Spanish MINECO: CSD2008-00005, The Spanish Ion Channel Initiative-CONSOLIDER INGENIO 2010, and BFU2012-39092-C02.Notes and references
- D. E. Clapham, L. W. Runnels and C. Strubing, Nat. Rev. Neurosci., 2001, 2, 387–396 CrossRef CAS PubMed.
- (a) L. Vay, C. Gu and P. A. McNaughton, Br. J. Pharmacol., 2012, 165, 787–801 CrossRef CAS PubMed; (b) D. Julius, Annu. Rev. Cell Dev. Biol., 2013, 29, 355–384 CrossRef CAS PubMed; (c) O. Radresa, H. Dahllöf, E. Nyman, A. Nolting, J. S. Albert and P. Raboisson, Open Pain J., 2013, 6(1), 137–153 CrossRef CAS; (d) M. Trevisani and R. Gatti, Open Pain J., 2013, 6(1), 108–118 CrossRef CAS; (e) Y. Kaneko and A. Szallasi, Br. J. Pharmacol., 2014, 171, 2474–2507 CrossRef CAS PubMed; (f) R. H. Straub, J. Mol. Med., 2014, 92, 925–937 CrossRef CAS PubMed.
- M. Camprubi-Robles, R. Planells-Cases and A. Ferrer-Montiel, FASEB J., 2009, 23, 3722–3733 CrossRef CAS PubMed.
- C. Morenilla-Palao, R. Planells-Cases, N. Garcia-Sanz and A. Ferrer-Montiel, J. Biol. Chem., 2004, 279, 25665–25672 CrossRef CAS PubMed.
- J. J. van Buren, S. Bhat, R. Rotello, M. E. Pauza and L. S. Premkumar, Mol. Pain, 2005, 1, 17 CrossRef PubMed.
- L. M. Broad, S. J. Keding and M. J. Blanco, Curr. Top. Med. Chem., 2008, 8, 1431–1441 CrossRef CAS PubMed.
- J. Huang, X. Zhang and P. A. McNaughton, Curr. Neuropharmacol., 2006, 4, 197–206 CrossRef CAS PubMed.
- K. Ueda, F. Tsuji, T. Hirata, M. Takaoka and Y. Matsumura, J. Cardiovasc. Pharmacol., 2008, 51, 513–520 CrossRef CAS PubMed.
- A. Babes, A. C. Ciobanu, C. Neacsu and R.-M. Babes, Curr. Pharm. Biotechnol., 2011, 12, 78–88 CAS.
- R. Ramachandran, E. Hyun, L. Zhao, T. K. Lapointe, K. Chapman, C. L. Hirota, S. Ghosh, D. D. McKemy, N. Vergnolle, P. L. Beck, C. Altier and M. D. Hollenberg, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7476–7481 CrossRef CAS PubMed.
- H. Xing, M. Chen, J. Ling, W. Tan and J. G. Gu, J. Neurosci., 2007, 27, 13680–13690 CrossRef CAS PubMed.
- S. E. Jordt, D. M. Bautista, H. H. Chuang, D. D. McKemy, P. M. Zygmunt, E. D. Hogestatt, I. D. Meng and D. Julius, Nature, 2004, 427, 260–265 CrossRef CAS PubMed.
- D. M. Bautista, P. Movahed, A. Hinman, H. E. Axelsson, O. Sterner, E. D. Hogestatt, D. Julius, S. E. Jordt and P. M. Zygmunt, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12248–12252 CrossRef CAS PubMed.
- Y. Karashima, K. Talavera, W. Everaerts, A. Janssens, K. Y. Kwan, R. Vennekens, B. Nilius and T. Voets, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 1273–1278 CrossRef CAS PubMed.
- D. del Camino, S. Murphy, M. Heiry, L. B. Barrett, T. J. Earley, C. A. Cook, M. J. Petrus, M. Zhao, M. D'Amours, N. Deering, G. J. Brenner, M. Costigan, N. J. Hayward, J. A. Chong, C. M. Fanger, C. J. Woolf, A. Patapoutian and M. M. Moran, J. Neurosci., 2010, 30, 15165–15174 CrossRef CAS PubMed.
- O. Fajardo, V. Meseguer, C. Belmonte and F. Viana, J. Neurosci., 2008, 28, 7863–7875 CrossRef CAS PubMed.
- H. Wei, A. Koivisto and A. Pertovaara, Neurosci. Lett., 2010, 479, 253–256 CrossRef CAS PubMed.
- J. Chen, S. K. Joshi, S. DiDomenico, R. J. Perner, J. P. Mikusa, D. M. Gauvin, J. A. Segreti, P. Han, X.-F. Zhang, W. Niforatos, B. R. Bianchi, S. J. Baker, C. Zhong, G. H. Simler, H. A. McDonald, R. G. Schmidt, S. P. McGaraughty, K. L. Chu, C. R. Faltynek, M. E. Kort, R. M. Reilly and P. R. Kym, Pain, 2011, 152, 1165–1172 CrossRef CAS PubMed.
- D. A. Barriere, J. Rieusset, D. Chanteranne, J. Busserolles, M.-A. Chauvin, L. Chapuis, J. Salles, C. Dubray and B. Morio, Pain, 2012, 153, 553–561 CrossRef CAS PubMed.
- A. Ferrer-Montiel, A. Fernandez-Carvajal, R. Planells-Cases, G. Fernandez-Ballester, J. Manuel Gonzalez-Ros, A. Messeguer and R. Gonzalez-Muniz, Expert Opin. Ther. Pat., 2012, 22, 999–1017 CrossRef CAS PubMed.
- (a) J. Chen, S. McGaraughty and P. R. Kym, TRPA1 in drug discovery, in TRP channels in drug discovery, ed. A. Szallazi and T. Bíró, Human Press, 2015, pp. 43–59 Search PubMed; (b) A. Fernández-Carvajal, G. Fernández-Ballester, R. González-Muñiz and A. Ferrer-Montiel, Pharmacology of TRP channels, in TRP Channels in sensory transduction, ed. R. Madrid and J. Bacigalupo, Springer, 2015, pp. 41–71 Search PubMed.
- P. Perez-Faginas, M. Teresa Aranda, M. Teresa Garcia-Lopez, L. Infantes, A. Fernandez-Carvajal, J. Manuel Gonzalez-Ros, A. Ferrer-Montiel and R. Gonzalez-Muniz, PLoS One, 2013, 8, e53231 CAS.
- M. Jesus Perez de Vega, M. Teresa Garcia-Lopez, L. Zaccaro, M. Royo, F. Albericio, A. Fernandez-Carvajal, A. Ferrer-Montiel and R. Gonzalez-Muniz, Molecules, 2010, 15, 4924–4933 CrossRef PubMed.
- G. Gerona-Navarro, R. Gonzalez-Muniz, A. Fernandez-Carvajal, J. M. Gonzalez-Ros, A. Ferrer-Montiel, C. Carreno, F. Albericio and M. Royo, ACS Comb. Sci., 2011, 13, 458–465 CrossRef CAS PubMed.
- L. Zaccaro, M. Teresa Garcia-Lopez, R. Gonzalez-Muniz, C. Garcia-Martinez, A. Ferrer-Montiel, F. Albericio and M. Royo, Bioorg. Med. Chem. Lett., 2011, 21, 3541–3545 CrossRef CAS PubMed.
- M. Angeles Bonache, B. Balsera, B. Lopez-Mendez, O. Millet, D. Brancaccio, I. Gomez-Monterrey, A. Carotenuto, L. M. Pavone, M. Reille-Seroussi, N. Gagey-Eilstein, M. Vidal, R. de la Torre-Martinez, A. Fernandez-Carvajal, A. Ferrer-Montiel, M. Teresa Garcia-Lopez, M. Martin-Martinez, M. Jesus Perez de Vega and R. Gonzalez-Muniz, ACS Comb. Sci., 2014, 16, 250–258 CrossRef PubMed.
- K. Sakakibara, M. Gondo and K. Miyazaki, WO 9303054 A1 19930218, 1993.
- K. E. B. Parkes, D. J. Bushnell, P. H. Crackett, S. J. Dunsdon, A. C. Freeman, M. P. Gunn, R. A. Hopkins, R. W. Lambert, J. A. Martin, J. H. Merrett, S. Redshaw, W. C. Spurden and G. J. Thomas, J. Org. Chem., 1994, 59, 3656–3664 CrossRef CAS.
- C. R. Theberge and C. K. Zercher, Tetrahedron, 2003, 59, 1521–1527 CrossRef CAS.
- U. Groselj, M. Zorz, A. Golobic, B. Stanovnik and J. Svete, Tetrahedron, 2013, 69, 11092–11108 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Protocol for the evaluation of compound 4b in the CFA-induced paw inflammation model and obtained results. See DOI: 10.1039/c5ra25709c |
| This journal is © The Royal Society of Chemistry 2016 |