Photoelectrochemical glucose biosensor based on a dehydrogenase enzyme and NAD+/NADH redox couple using a quantum dot modified pencil graphite electrode†
Abstract
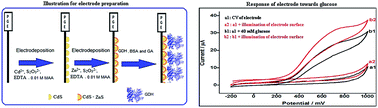
A simple, disposable and economical modified electrode was prepared by electrodeposition of hybrid quantum dots (ZnS–CdS) onto a pencil graphite electrode (PGE) surface and subsequent immobilization of glucose dehydrogenase (GDH) onto the quantum dot modified electrode (GDH/ZnS–CdS/PGE). The prepared electrode was effectively used for the photoelectrochemical determination of glucose in a flow injection analysis (FIA) system using a new home-made flow cell which was designed for PGEs for the first time. Results from the cyclic voltammetric and FI amperometric measurements revealed that the GDH/ZnS–CdS/PGE is capable of signaling photoelectrocatalytic activity involving NADH when the surface of the GDH/ZnSr–CdS/PGE is irradiated with a light source with a fiber optic cable (250 W halogen lamp). The currents of NADH produced by the enzymatic reaction in the photoamperometric FIA system under optimized conditions (carrier stream: 0.1 M phosphate buffer solution (pH 7.0) containing 1.0 M KCl and 10.0 mM NAD+, applied potential: +0.8 V vs. Ag/AgCl/KCl(sat.); flow rate: 0.6 mL min−1, sample loop: 100 μL; transmission tubing length: 10 cm) were linearly correlated with the glucose concentration. Calibration curves were obtained for glucose concentrations within a range from 0.2 to 8.0 mM. The detection limits were found to be 0.09 and 0.05 mM for the amperometric and photoamperometric methods, respectively. The relative standard deviations (n = 7) for 0.5 mM glucose were 4.5% and 3.5% from the photoamperometric and amperometric results respectively. The photoelectrochemical biosensor was applied to real samples successfully. The results with this biosensor showed good selectivity, repeatability and sensitivity for monitoring glucose in amperometric and photoamperometric FIA studies.

 Please wait while we load your content...
Please wait while we load your content...