Measuring the interaction between a pair of emulsion droplets using dual-trap optical tweezers
Abstract
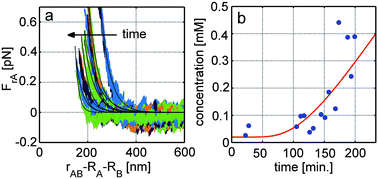
Optical tweezers have been used to investigate the dependance of electrostatic inter-particle forces on separation, in systems consisting of pairs of either model silica beads or emulsion droplets. Measurements were carried out as a function of ionic strength and, at salt concentrations where the Debye length was larger than the standard deviation of Brownian fluctuations of the particles in the traps, results were found to agree reasonably well with the predictions of DLVO theory. Experiments were also carried out where the salt concentration of the environment was changed in real-time while interactions were continuously measured. Specifically, single pairs of particles or emulsion droplets were held in a microfluidic channel in close proximity to an interface created between milliQ water and a 5 mM NaCl solution. Changes in the force–separation curves were measured as a function of time and used to monitor changes in the Debye length, and thus the local salt concentration, as ions diffused away from the interface. The results were shown to be consistent with expectations based on a relevant diffusion equation.

 Please wait while we load your content...
Please wait while we load your content...