A facile and practical copper diacetate mediated, ligand free C–N cross coupling of trivalent organobismuth compounds with amines and N-heteroarenes†
Abstract
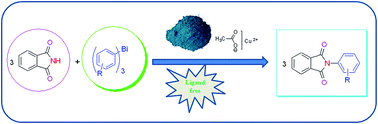
In present work, an efficient Cu(OAc)2·H2O catalyzed protocol in the absence of any additional ligand has been developed for the N-arylation of amines and nitrogen containing heterocycles using trivalent organobismuth reagents under mild conditions. This protocol tolerates a variety of functional groups on amines and the organobismuth reagent with a high degree of chemoselectivity.

 Please wait while we load your content...
Please wait while we load your content...