Visible light photocatalysis with CBr4: a highly selective aerobic photooxidation of methylarenes to aldehydes†
Shubhangi Tripathi,
Sachchida N. Singh and
Lal Dhar S. Yadav*
Green Synthesis Lab, Department of Chemistry, University of Allahabad, Allahabad 211 002, India. E-mail: ldsyadav@hotmail.com; Fax: +91-532-246-0533; Tel: +91-532-250-0652
First published on 26th January 2016
Abstract
A metal-free, operationally simple and efficient means of aerobic photooxidation of methylarenes to aromatic aldehydes at room temperature employing visible light photocatalysis with CBr4 is reported. The reaction is highly selective as it proceeds without any over oxidation to carboxylic acids. The utilisation of visible light and molecular oxygen is inexpensive, readily available, non-toxic and the sustainable reagents make the protocol compatible with green chemistry demands. A plausible mechanism for the formation of aldehydes from methylarenes is also provided.
Introduction
The fundamental objective of chemists is to develop new sustainable routes for chemical transformations having significant conceptual and practical interest under mild reaction conditions.1 In this context, visible light-mediated photoredox catalysis has emerged as a state-of-the-art alternative to advance this goal.2 In recent years, visible light-induced synthetic methodologies have received central attention from various research groups, especially because of its eco-compatibility, easy availability, safe handling and everlasting abundance as an energy source.3 Consequently, a number of synthetic protocols have been developed that utilise the powerful photocatalytic properties of highly expensive and potentially toxic ruthenium and iridium complexes. Therefore, the development of synthetically useful chemical transformations employing inexpensive visible light photocatalysts would be warmly welcomed, especially in terms of economical and environmental issues.Among various methods, oxidation reactions having unique advantage to activate inert bonds, especially C–H bonds, play a pivotal role in the chemical industry for designing and realising the chemical intermediates and fine chemical specialties.4,5 However, selectivity of oxidation is still an open challenge for chemists. Similarly, carbonyl compounds, the vital substrates in organic synthesis,6,7 are still synthesized by non-eco-compatible methods with very low atom economy involving copious amount of waste generation. Hence, selective oxidation of methylarenes to synthetically important carbonyl compounds is quite demanding concerning both reactivity and selectivity issues.
Traditionally, inorganic oxidizing agents like chromates, permanganates and cerrates, employed in stoichiometric amounts, suffer from noxious heavy-metal waste generation and their outlay.8 Therefore, development of a cleaner and cost-effective methodology would be a valuable contribution to this industrially important field.
In contrast to traditional methods several transition-metal based catalytic processes using selenium,9 silver10 and copper11 metal catalysts have also been reported for selective oxidation of methylarenes. Though these metal catalysts are very effective for selective oxidation, most of them are very expensive and hazardous to the environment. To overcome the hazards associated with metal catalysts, several non-metallic catalytic pathways for selective oxidation of methylarenes were reported viz. 9,10-dicyanoanthracene/methyl viologen and oxygen mediated photocatalysis12 and enzyme laccase/ABTS,8 DDQ/NBS,13 H2O2/HBr,14 Br2/DMSO15 or IBX/DMSO16 catalysis but these processes also suffer from one or more drawbacks of substrate limitations, handling problems, poor selectivity and yield issues. Hence, development of a metal-free, environmentally benign, cost effective and sustainable method for selective oxidation of methylarenes is necessarily a welcome move.
Recently, a stoichiometric amount of CBr4 has been used to generate bromine free radicals under visible light irradiation in several synthetically useful reactions.18d,19b We reasoned that a photoreaction employing CBr4 as a source of bromine radicals and producing HBr as a by-product could be rendered catalytic with respect to CBr4 in the presence of an oxidant which in situ regenerates the bromine free radical from HBr.
Inspired by the aforementioned facts and our continued efforts to devise metal-free efficient synthetic routes,18 we hypothesised visible light-mediated one-pot selective oxidation of methylarenes up to aromatic aldehydes only employing CBr4 as a photocatalyst and molecular oxygen as an oxidant (Scheme 1c). Implication of visible light and molecular oxygen as reagents makes the protocol greener, more viable and sustainable than existing methodologies. Moreover, visible light is a traceless reagent and oxygen is believed to generate water as the sole by-product. The present protocol on selective oxidation of methylarenes being inexpensive, metal-free, employing economical acquisition of reagents would be a valuable addition to this field.7 A plausible mechanistic pathway for the selective oxidation of methylarenes to aldehydes is given in Results and Discussion Section.
Results and discussion
To realise our hypothesis and determine the optimal reaction conditions, we materialised the study with a model reaction of toluene (1a, 1 mmol) with catalytic amount of CBr4 (2, 10 mol%) in acetonitrile under an oxygen atmosphere (O2 balloon) and visible light irradiation using 18 W CFL (380–740 nm) for 2 h at rt. To our delight, the desired product benzaldehyde (3a) was obtained as the sole product in an excellent yield of 89%. With this encouraging result, we performed a series of control and screening experiments and firstly observed that the reaction did not proceed in the dark (Table 1, entry 2). Similarly, the absence of CBr4 under the reaction conditions leads to the same conclusion (Table 1, entry 3). These results suggest the necessity of both light and CBr4 for the reaction. Next, to determine the role of oxygen, the screening was performed under degassed condition and an inert argon atmosphere (Table 1, entries 4 and 5) and the results revealed that the molecular oxygen is equally essential for the present oxidation reaction. The system also worked under an air atmosphere instead of an oxygen balloon but delivered a considerably lower yield of 3a (Table 1, entry 6). Cumulatively, on decreasing the amount of CBr4 from 10 mol% to 5 mol%, the yield of 3a was significantly reduced (Table 1, entry 1 vs. 7). However, use of 15 mol% CBr4 did not affect the yield of the product (Table 1, entry 8). Other bromine sources, viz. NBS, and BDMS (bromodimethylsulfonium bromide) did not work satisfactorily under the present reaction conditions (Table 1, entries 1 vs. 9 and 10).| Entry | Reaction conditions | Irradiation time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: toluene (1a, 1.0 mmol) and CBr4 (2, 10 mol%) in CH3CN (3 mL) were irradiated under a molecular oxygen atmosphere (O2 balloon) at rt using 18 W CFL for 2–18 h.b Isolated yield of the product 3a; nd = not detected.c Reaction was carried out in the dark.d Reaction was carried out under degassed condition.e Reaction was carried out under an argon atmosphere. | |||
| 1 | CBr4 (10 mol%), O2 balloon, 18 W CFL | 2 | 89 |
| 2c | CBr4 (10 mol%), O2 balloon, in the dark | 8 | nd |
| 3 | Without CBr4, O2 balloon, 18 W CFL | 8 | nd |
| 4d | CBr4 (10 mol%), degassed, 18 W CFL | 8 | nd |
| 5e | CBr4 (10 mol%), argon, 18 W CFL | 8 | nd |
| 6 | CBr4 (10 mol%), air, 18 W CFL | 4 | 62 |
| 7 | CBr4 (5 mol%), O2 balloon, 18 W CFL | 4 | 45 |
| 8 | CBr4 (15 mol%), O2 balloon, 18 W CFL | 2 | 89 |
| 9 | NBS (10 mol%), O2 balloon, 18 W CFL | 18 | 26 |
| 10 | BDMS (10 mol%), O2 balloon, 18 W CFL | 18 | 6 |
Given the importance of solvent activity in chemical transformations, we performed a series of experiments for optimization. The results reveal the superiority of acetonitrile over the other tested solvents, viz. CH3OH, CH3CH2OH, tBuOH, hexane and THF (Table 2, entry 1 vs. 2–6).
| Entry | Solvent | Time (h) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: toluene (1a, 1.0 mmol) and CBr4 (2, 10 mol%) in a solvent (3 mL) were irradiated under a molecular oxygen atmosphere at rt using 18 W CFL for 2–8 h. | |||
| 1 | CH3CN | 2 | 89 |
| 2 | CH3OH | 8 | 40 |
| 3 | CH3CH2OH | 8 | 38 |
| 4 | tBuOH | 8 | 27 |
| 5 | Hexane | 8 | 35 |
| 6 | THF | 8 | 18 |
Having established the optimal reaction conditions for visible light-mediated oxidation of methylarenes, we surveyed the generality and scope of the present protocol over a wide range of methylarenes (Table 3).
In general, methylarenes bearing either an electron-donating (Me or OMe) or an electron-withdrawing substituent (NO2, F, Cl, Br, I, CN, MeSO2, PhSO2, CONH2, COOEt or COPh) are well tolerated in the present methodology and afford carbonyl compounds in good to excellent yields of 75–96%. However, methylarenes bearing an electron-donating substituent on the aromatic ring appear to react faster and afford marginally higher yield than those bearing an electron-withdrawing group. Also, evaluation of aliphatic hydrocarbons in the present methodology does not lead to the satisfactory results probably due to the far less stability of an alkyl radical than a benzyl radical.
Congruent with the above observations and the literature precedents,17,19 a plausible mechanism for the visible light and CBr4-promoted selective oxidation of methylarenes to aromatic aldehydes is depicted in Scheme 3. The mechanism might follow a radical pathway, as on addition of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxyl), a traditional radical scavenger, the reaction was quenched (Scheme 2) and that the formation of benzyl-TEMPO adduct was confirmed by its MS (HRMS (EI) calcd for C16H25NO: 247.1936, found 247.1934). On irradiation with visible light using 18 W CFL under a molecular oxygen environment, CBr4 generates the bromine radical, which reacts with methylarene 1 to form resonance stabilised benzyl radical 4 along with HBr. The benzyl radical 4 combines with O2 to generate peroxy radical 5. The peroxy radical 5 abstracts hydrogen atom from HBr and regenerates the bromine radical along with the hydroperoxide 6, which eliminates H2O to afford the desired product 3.
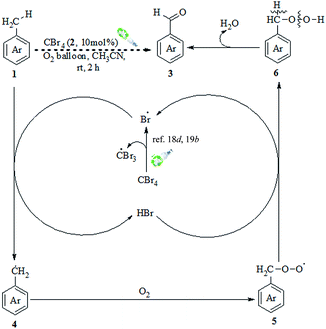 | ||
| Scheme 3 Plausible mechanism for the visible light-promoted aerobic oxidation of methylarenes to aldehydes. | ||
Conclusion
In conclusion, we have developed a novel, highly efficient, metal-free, one-pot synthesis of aromatic aldehydes by aerobic oxidation of methylarenes employing visible light photocatalysis with CBr4. Notably, water is the only by-product in the reaction. The protocol overrides the other methods for highly selective oxidation of methylarenes only up to the aldehyde stage. Advantageously, it utilises visible light and molecular oxygen as clean, inexpensive and sustainable reagents and does not require any additives, heating or inert conditions.Acknowledgements
We sincerely thank SAIF, Punjab University, Chandigarh, for providing microanalyses and spectra.References and notes
- C. Dai, J. M. R. Narayanam and C. R. J. Stephenson, Nat. Chem., 2011, 3, 140 CrossRef CAS PubMed.
- For leading reviews on visible light photoredox catalysis, see: (a) K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785 CrossRef CAS PubMed; (b) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed; (c) F. Teply, Collect. Czech. Chem. Commun., 2011, 76, 859 CrossRef CAS; (d) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (e) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (f) Y. Xi, H. Yia and A. Lei, Org. Biomol. Chem., 2013, 11, 2387 RSC; (g) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (h) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 CrossRef PubMed.
- (a) G. Ciamician, Science, 1912, 36, 385 CAS; (b) A. Albini and M. Fagnoni, ChemSusChem, 2008, 1, 63 CrossRef CAS PubMed.
- (a) J. A. Labinger and J. E. Bercaw, Nat. Chem., 2002, 417, 507 CrossRef CAS PubMed; (b) R. A. Sheldon and H. van Bekkum, Fine Chemicals through Heterogeneous Catalysis, Wiley-VCH, Weinheim, 2001, p. 1 Search PubMed; (c) J. M. Thomas, R. Raja, G. Sankar and R. G. Bell, Nature, 1999, 398, 227 CrossRef CAS.
- (a) A. D. Sadow and T. D. Tilley, Angew. Chem., Int. Ed., 2003, 42, 803 CrossRef CAS PubMed; (b) C. Limberg, Angew. Chem., Int. Ed., 2003, 42, 5932 CrossRef CAS PubMed; (c) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 CrossRef CAS PubMed; (d) B. B. Sarma, I. Efremenko and R. Neumann, J. Am. Chem. Soc., 2015, 137, 5916 CrossRef CAS PubMed.
- (a) F. Bruhne and E. Wright, Industrial Organic Chemicals: Starting Materials and Intermediates – An Ullmann's Encyclopedia, Wiley-VCH, Weinheim, Germany, 1999, p. 673, and references cited therein Search PubMed; (b) R. A. Sheldon, I. W. C. E. Arends, G. J. Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774 CrossRef CAS PubMed.
- (a) Y. Xie, W. Mo, D. Xu, Z. Shen, N. Sun, B. Hu and X. Hu, J. Org. Chem., 2007, 72, 4288 CrossRef CAS PubMed; (b) C. X. Miao, L. N. He, J. L. Wang and F. Wu, J. Org. Chem., 2010, 75, 257 CrossRef CAS PubMed; (c) M. Tabata, K. Moriyama and H. Togo, Eur. J. Org. Chem., 2014, 3402 CrossRef CAS.
- A. Potthast, T. Rosenau, C. L. Chen and J. S. Gratzl, J. Org. Chem., 1995, 60, 4320 CrossRef CAS.
- (a) D. H. R. Barton, R. A. H. F. Hui, D. J. Lester and S. V. Ley, Tetrahedron Lett., 1979, 20, 3331 CrossRef; (b) D. H. R. Barton and T. Wang, Tetrahedron Lett., 1994, 35, 5149 CrossRef CAS.
- (a) R. G. R. Bacon and J. R. Doggart, J. Chem. Soc., 1960, 1332 RSC; (b) H. Firouzabadi and P. Salehi, Synth. Commun., 1991, 21, 1121 CrossRef CAS.
- (a) M. V. Bhatt and P. T. Perumal, Tetrahedron Lett., 1981, 22, 2605 CrossRef CAS; (b) M. P. Gore, S. J. Gould and D. D. Weller, J. Org. Chem., 1992, 57, 2774 CrossRef CAS.
- J. Santamaria and R. Jroundi, Tetrahedron Lett., 1991, 32, 4291 CrossRef CAS.
- H. Lee and R. G. Harvey, J. Org. Chem., 1988, 53, 4587 CrossRef CAS.
- M. Ghaffarzadeh, M. Bolourtchian, K. Taber-Heydar, I. Daryaei and F. Mohsenzaheh, J. Chem. Sci., 2009, 121, 177 CrossRef CAS.
- M. Ghaffarzadeh, M. Bolourtchian, M. Gholamhosseni and F. Mohsenzaheh, Appl. Catal., A, 2007, 333, 131 CrossRef CAS.
- K. C. Nicolaou, T. Montagnon, P. S. Baran and Y. L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245 CrossRef CAS PubMed.
- S. Gazi and R. Ananthkrishnan, RSC Adv., 2012, 2, 7781 RSC.
- (a) A. K. Yadav, V. P. Srivastava and L. D. S. Yadav, New J. Chem., 2013, 37, 4119 RSC; (b) A. K. Yadav and L. D. S. Yadav, Tetrahedron Lett., 2014, 55, 2065 CrossRef CAS; (c) T. Keshari, V. K. Yadav, V. P. Srivastava and L. D. S. Yadav, Green Chem., 2014, 16, 3986 RSC; (d) T. Keshari, V. P. Srivastava and L. D. S. Yadav, RSC Adv., 2014, 4, 5815 RSC; (e) R. Chawla, A. K. Singh and L. D. S. Yadav, Eur. J. Org. Chem., 2014, 2032 CrossRef CAS; (f) A. K. Singh, R. Chawla and L. D. S. Yadav, Tetrahedron Lett., 2015, 56, 653 CrossRef CAS; (g) S. Tripathi, S. N. Singh and L. D. S. Yadav, Tetrahedron Lett., 2015, 56, 4211 CrossRef CAS.
- (a) K. Ohkubo, K. Mizushima and S. Fukuzumi, Res. Chem. Intermed., 2013, 39, 205 CrossRef CAS; (b) Y. Nishina, B. Ohtani and K. Kikushima, Beilstein J. Org. Chem., 2013, 9, 1663 CrossRef PubMed.
- (a) R. L. Shriner, R. C. Fusion, D. Y. Curtin and T. C. Morrill, The Systematic Identification of Organic Compounds, 6th ed, 1980 Search PubMed; (b) S. T. M. Ali, E. Lourdusamy and S. Arumugan, J. Org. Chem., 2006, 71, 5043 CrossRef PubMed; (c) C. Lin and T. Lu, Tetrahedron, 2010, 66, 9688 CrossRef CAS; (d) A. N. Bandna and P. Das, Tetrahedron Lett., 2011, 52, 4954 CrossRef; (e) K. Layek, H. Maheswaran, R. Arundhathi, M. L. Kantam and S. K. Bhargava, Adv. Synth. Catal., 2011, 353, 606 CrossRef CAS; (f) Y. Huang, X. Fang, X. Lin, W. He, Y. Yuan, Z. Weng, H. Li and K. Huang, Tetrahedron, 2012, 68, 9949 CrossRef CAS; (g) P. Wang, J. Yang, L. Li, H. Hu, J. Cai, C. Sun and M. Jin, Tetrahedron Lett., 2013, 54, 533 CrossRef CAS; (h) I. Masataka, M. Katsuhiko and T. Hideo, Tetrahedron, 2013, 69, 2961 CrossRef; (i) Z. Li, W. Zhu, J. Bao and X. Zou, Synth. Commun., 2014, 44, 1155 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26623h |
| This journal is © The Royal Society of Chemistry 2016 |





