IDO as a drug target for cancer immunotherapy: recent developments in IDO inhibitors discovery
Shan Qian*a,
Man Zhanga,
Quanlong Chena,
Yanying Hea,
Wei Wanga and
Zhouyu Wang*b
aDepartment of Pharmaceutical Engineering, Xihua University, Chengdu 610039, P. R. China. E-mail: qians33@163.com
bDepartment of Chemistry, Xihua University, Chengdu 610039, P. R. China. E-mail: zhouyuwang77@gmail.com
First published on 5th January 2016
Abstract
The indoleamine 2,3-dioxygenase (IDO) mediated kynurenine pathway of tryptophan degradation is identified as an important immune effector pathway in tumor cells to escape a potentially effective immune response. IDO affects the differentiation and proliferation of T cells, triggering downstream signaling through GCN2, mTOR and AhR. Therefore, IDO is an attractive target for cancer immunotherapy. IDO inhibitors exhibit potent anticancer activities and might be very useful in combination with chemotherapy, radiotherapy or immunotherapy to trigger the rapid regression of aggressive tumors. However, the development of IDO pharmacological inhibitors has been a challenging work. This review highlights recent advances (2010–2015) in research related to the role of IDO in immune escape and pathogenic inflammation in cancer, and novel small-molecule IDO inhibitors with an emphasis on their chemical structures and modes of action.
1. Immunotherapy is an exciting strategy of cancer therapy
The interactions between the immune system and the developments of tumors are dynamic and complex. On one hand, inflammation suppresses the immune responses to actually promote tumor progression; on the other hand, tumor cells are also able to evade or subvert the immune response upon the pressure of immune surveillance. It will be effective and critical to breach immune suppressive mechanisms established by tumor cells in the treatment of cancer patients. In 2010, the first therapeutic vaccine sipuleucel-T was approved for the treatment of metastatic prostate cancer by the Food and Drug Administration (FDA). Since then, immunotherapeutic approaches have been unleashed and are rapidly expanding worldwide in the field of cancer research. In 2011, the first cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody, ipilimumab, was approved for the treatment of metastatic melanoma (MM). Programmed cell death 1 and its ligand (PD-1 and PD-L1) antibodies as new generations of ‘immune checkpoint blockade’ are now under intense clinical investigation in MM and non-small-cell lung cancer (NSCLC). PD-1/PD-L1 antibodies nivolumab and pembrolizumab are evaluated as the blockbuster drugs in the near future. Immunotherapy technologies of chimeric T cell antigen receptors (CAR-T) have achieved remarkable success in the treatment of leukemia and solid tumors (pancreatic carcinoma). Cancer immunotherapy has been nominated for the ‘Year Breakthrough of Cancer Research 2013’ by Science magazine.1 More than 50 phase III trials are currently on-going in cancer immunotherapy. Because of distinguished effectiveness and novelty, cancer immunotherapies promise to be an innovation in the treatment of cancer following surgical treatment, chemotherapy, radiotherapy, and targeted therapy.2. IDO is a key mediator of immune escape and pathogenic inflammation in cancer
Indoleamine 2,3-dioxygenase (IDO), an extrahepatic tryptophan catabolising enzyme encoded by the IDO1 gene, degrades the essential amino acid tryptophan (TRP) to kynurenine (KYN), known as the initial and rate limiting step of the kynurenine pathway. Expression of the IDO1 gene by tumor cells or host APCs can inhibit tumor-specific effector CD8+ T cells and enhance the suppressor activity of T regulatory cells (Tregs). The IDO-mediated catabolism of TRP has been identified as an important immune effector pathway in tumor cells to escape a potentially effective immune response, which is induced by innate and adaptive immune responses.IDO affects the differentiation and proliferation of T cells, triggering downstream signaling through GCN2, mTOR and AhR (Fig. 1). First, TRP degradation by IDO may cause local TRP deprivation in the tissue microenvironment that leads to the accumulation of TRP-tRNA in uncharged conformation. General control non-derepressible 2 (GCN2) is a stress-response kinase containing an allosteric regulatory site that responds to uncharged tRNA. The activation of GCN2 kinase leads to phosphorylation and attenuation of its downstream target, eukaryotic translation initiation factor-2α (eIF-2α) kinase. Phosphorylated eIF-2α prevents the readout of most RNA transcripts and limits protein translation in response to this condition.2 Second, another signaling molecule inhibited due to the absence of TRP is the master metabolic regulator mTOR (mTORC1), which is the regulatory target of the master amino acid-sensing kinase 1 (GLK1). Local TRP degradation can block GLK1. The GLK1 blockade suppresses mTORC1 and is sufficient to trigger autophagy. IDO suppresses mTORC1 as an analogue of the mTOR inhibitor rapamycin to trigger autophagy and anergize T cells in the tumor microenvironment.3 Thus, TRP depletion in the local microenvironment consumed by IDO can act as a potent regulatory signal via two distinct molecular stress-response pathways, one of which is activated by amino acid insufficiency signaling (GCN2/eIF-2α) and the other that is blocked by amino acid sufficiency signaling (GLK1/mTORC1). Third, IDO-mediated catabolism of TRP produces KYN, an endogenous ligand of the aryl hydrocarbon receptor (AhR). The AhR is a ligand-activated transcription factor responding to xenotoxins such as dioxin and involved in embryogenesis, transformation and tumorigenesis. KYN binding to AhR is essential to promote the differentiation of Tregs that suppress antitumor immune responses. The activation of AhR helps tumors program a pathogenic inflammation in their microenvironment to escape from immunosurveillance toward immune escape. AhR signaling accounts for the interactions of immunology, toxicology and cancer development and may explain why TRP consumption helps pathogenic inflammatory programming and drives malignant progression4,5 (Fig. 2).
The role of IDO in cancer pathogenesis is more complex than simply acting as a modifier of immune tolerance. Genetic studies in IDO-null mice indicated that IDO contributes to establish the pathogenic inflammatory state, which is a hospitable environment for tumor development and metastatic progression.6 As shown in the classical multistage protocol of DMBA+TPA-induced carcinogenesis, the induction of IDO was integral to the pathogenic inflammatory microenvironment, even in the absence of cancer. By the immunoediting definition, there are some nascent tumor cells at least to induce IDO. However, TPA treatment alone was sufficient to induce IDO as an initiating agent to drive the development of tumors because these mice of DMBA treatment were never exposed to tumor initiation.6 Another observation showed that the inflammation in IDO-deficient mice does not run rampant and is elicited by treatment with a pro-inflammatory agent.7 In conjunction with results described above, IDO elevation is an early event driven by inflammation, and IDO contributes to the pathogenic chronic inflammation of the tissue microenvironment.
IDO is silent in most tissues but highly active in the placenta required for the maternal immune tolerance of the fetus. Many types of cancer cells and tumor environmental cells can express IDO in a constitutive manner or strongly induced by inflammatory mediators such as interferon-gamma (IFN-γ) when the host responses against the tumor and produces inflammatory environment. The deregulation of IDO in tumor cells is due to cancer suppressive gene called bridging integrator 1 (Bin1). Bin1 is a c-myc interacting protein with the features of the tumor suppressor that interacts with the N-terminal of c-Myc protein to neutralize its transformation and transactivation effect.8 Clinical observations have suggested that frequent loss or attenuation of Bin1 and high IDO expression are seen in various cancers, including advanced breast cancer, prostate cancer, melanoma, astrocytoma, neuroblastoma, lymphocytic leukemia and colon cancer, and are associated with a poorer clinical prognosis in patients with a variety of malignancies.9,10 Bin1 can restrict tumor outgrowth and limit tumor immune tolerance through its effect on the down regulation of IDO expression. On the contrary, mouse knockout studies indicated that a deletion of the Bin1 results in the STAT1 and NF-κB dependent super-expression of IDO following IFN-γ stimulation.11 Bin1 knockout mice are expected to result in large tumor growth when they are transduced with c-myc and mutant ras oncogenes. IDO inhibition can prevent tumor growth in these mice, suggesting a direct link between the Bin1 and IDO activity.11
3. Therapeutic strategies and challenges of IDO inhibitors in cancer immunotherapy
Targeting the IDO pathway via inhibition of the IDO enzyme or blocking its downstream signaling effects is a prime target for small-molecule immunomodulatory drugs in cancer. It has also been widely recognized that IDO inhibitors might be useful in combination with traditional chemotherapeutic drugs or therapeutic cancer vaccines. Damaged or dying tumor cells exposed to chemotherapeutic regimens can express many antigens to activate immune responses against the tumor. However, this almost never triggers a curative immune response against an established human tumor, because the tumor can rapidly reestablish tolerance following each cycle of chemotherapy by upregulating immunological checkpoints, including IDO.12 In addition, chemotherapy induces the transient lymphopenia and cytokine-rich environment that may promote T cells to break their tolerance to homeostatic recovery.13 Finally, chemotherapy can also deplete and blunt regulatory T cells, which can generate a short-lived but beneficial anti-tumor immune response.14 Thus, inhibiting IDO in the period of post-chemotherapy could promote T cells to more receptively break tolerance and potentially enhance the effectiveness of chemotherapeutics by active immunotherapy.Multiple immune inhibitory mechanisms are present concurrently in the tumor microenvironment, so single agent treatments targeting one immunological checkpoint with optimal efficiency are difficult to achieve. Allison et al.15 indicated that IDO is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The IDO blockade strongly synergizes with CTLA-4 antibody, ipilimumab, which achieved a striking delay in B16 melanoma tumor growth and increased overall survival. Spranger et al.16 also reported that multiple inhibitions of IDO, CTLA-4 and PD-1/PD-L1 showed greater effect in activating the immune system and inhibiting tumor growth than either treatment alone. The responses showed more pronounced activation (proliferation + cytokine production) of intratumoral CD8+ T cells. These data provided a strong incentive to explore combination clinical therapies using IDO inhibitors regardless with IDO expression by the tumor cells.
Recently, the development of IDO inhibitors are ongoing intensively in academia and pharmaceutical companies, including Pfizer Pharmaceuticals Ltd, Roche, Bristol-Myers Squibb, and three small-molecule compounds (indoximod, epacadostat and GDC-0919) have entered clinical trials.17 As a kynurenine pathway inhibitor, indoximod (NLG8189) improves the efficacy of multiple chemotherapeutics agents (paclitaxel, docetaxel, temozolomide and gemcitabine), and some immunological checkpoints mediators (sipuleucel-T and ipilimumab) in phase I/II clinical studies for metastatic breast cancer, metastatic melanoma, non-small cell lung cancer, primary malignant brain tumors, metastatic pancreatic cancer, as well as metastatic prostate cancer (source http://www.clinicaltrials.gov). Epacadostat (INCB024360) was obtained following a high throughput screening (HTS) of Incyte's corporate collection (IC50 = 67 nM).18 It promotes T and natural killer (NK)-cell growth, increases IFN-γ production, and reduces conversion to Treg-like cells.19 Epacadostat suppresses KYN generation and tumor growth in immunocompetent, but not immunodeficient mice.19 In mice bearing CT26 colon carcinoma, epacadostat also inhibits the growth of IDO-expressing tumors.20 Epacadostat is evaluated at present mainly in phase I/II clinical combination drug trials in gynecological and peritoneal cancers, melanoma, malignant solid tumor, lymphoma, breast, lung, as well as renal cell cancers with CTLA-4 and PD-1/PD-L1 antibodies. GDC-0919 (NLG919) was obtained by rational structural design based on the X-ray crystal structure for IDO complexed with 4-phenyl-imidazole (PIM). It is a strong competitive IDO inhibitor and potently inhibits IDO pathway in vitro and in cellular assays (Ki = 7 nM, EC50 = 75 nM). Treatment with GDC-0919, upon vaccination of B16F10 tumor-bearing mice, resulted in an increase in the T effector cell response, leading to improved anti-tumor efficacy (∼95% reduction in tumor volume).21 Combined treatment with GDC-0919 and chemo-radiation therapy enhanced survival in mice bearing intracranial glioblastoma tumors. Chemotherapy treatment alone resulted in collections of perivascular leukocytes within the tumor microenvironment, but no complement deposition. Adding the IDO-blockade led to the upregulation of vascular cell adhesion molecule-1 (VCAM-1) on vascular endothelium within tumors and further led to widespread complement deposition at sites of tumor growth.22 In the phase Ia clinical trial for the treatment of advanced-stage solid tumors, GDC-0919 demonstrated the high dose tolerance (well tolerated up to 800 mg BID on a 21/28 day cycle), but revealed that single-agent therapy with an IDO inhibitor failed to cause tumor eradication and prevent disease progression (best response was limited to stable disease in 7 out of 17 patients). GDC-0919 is now evaluated in phase Ib in combination with PD-L1 inhibitor atezolizumab.23
4. Structure-based IDO inhibitors design
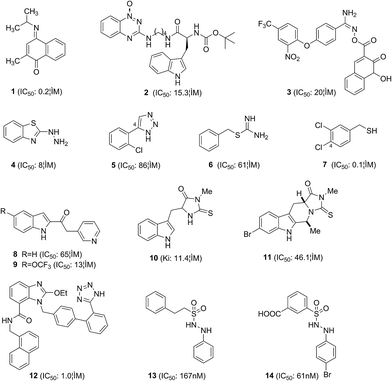
The crystal structure of IDO bound to the ligand PIM published by Sugimoto et al. in 2006 [Protein Data Bank (PDB) file“2D0T”,24 Fig. 3] opened the door for the structure-based in silico design of IDO inhibitors. IDO is a monomeric heme-containing enzyme. In the PIM-bound X-ray structure, the ligand is bound in a deep binding site with one imidazole nitrogen coordinated to the Fe iron at the distance of 2.1 Å and its phenyl ring is inside a hydrophobic pocket (pocket A). The PIM binding sites consist of residues Tyr126, Cys129, Val130, Phe163, Phe164, Ser167, Leu234, Gly262, Ser263, Ala264, and the heme ring. Possible hydrogen bonding sites are the Cys129-SH, Ser167-OH, Gly262-CO, Ala264-NH and the heme 7-propionate group. In 2014, two crystal structures of IDO bound to the larger ligands Amg-1 (PDB file “4PK5”) and imidazothiazole compound (PDB file “4PK6”) were published25 (Fig. 3). Another hydrophobic pocket (pocket B) at the entrance of pocket A is provided by multiple residues and the heme ring. In addition to occupy pocket A, the larger ligands can also interact with residues located at pocket B, containing Phe226, Arg231, Ser235, Phe291, Ile354, and Leu384. Moreover, the additional hydrogen bond with the side chain of Arg231 is possible. The ligands occupied both pocket A and pocket B, which may obtain better inhibitory activities (Fig. 4).Röhrig et al.26 investigated the binding modes of all known IDO inhibitors via docking algorithm EA Dock and developed a pharmacophore model to devise new compounds to be tested for IDO inhibition. A fragment-based approach was also used to design and to optimize small organic molecule inhibitors. Both approaches yielded several novel small-molecular inhibitor scaffolds and the most active compound 1 showed nanomolar inhibitory activity. On the basis of the binding modes between IDO and these ligands, they concluded that an ideal IDO inhibitor should contain the following features (i) an aromatic ring with at least two cycles, to fill into pocket A (almost all known IDO inhibitors comply with this rule); (ii) an atom with a free electron pair (such as oxygen, sulfur, or nitrogen) that can coordinate to the Fe iron (ligands not obeying this rule generally show low inhibitory activities); (iii) groups in larger ligands that can form van der Waals interactions with the binding sites in the pocket B; and (iv) groups in larger ligands that can form hydrogen bond to Ser167, Gly262, Ala264, and Arg231 or to the heme 7-propionate.26 In the same year, a series of hypoxia-targeting novel IDO hybrid inhibitors have been designed and synthesized by Nakashima et al.27 Among these compounds, L-Trp-tirapazamine hybrid 2 was able to bind the enzyme–substrate complex and showed potent IDO inhibitory activity with an IC50 value of 15.3 μM. Smith et al.28 developed three pharmacophores to search for novel IDO inhibitors, coupled with refinement of hits through Kier flexibility scoring and “What-If” docking analysis. Eighteen compounds were tested in vitro, yielding compound 3 (IC50: 20 μM) with an ester imidamide linker between two aromatic regions, which have much greater interactions with the active site compared to many of the simple IDO inhibitors reported to date. Fragment screening has the capacity to identify novel IDO inhibitors, including those that cannot be identified by enzymatic screening. 2-Hydrazinobenzothiazole was identified as a strong IDO inhibitor by screening the Zenobia fragment library. It forms a co-ordinate bond with the heme iron to stabilize the complex through hydrazine rather than through sulphur in the benzothiazole ring. Phenylhydrazine was 32-fold more potent (IC50: 0.25 μM) than 2-hydrazinobenzothiazole (4) (IC50: 8.0 μM).29 The enzyme assay indicated that the hydrazine interaction is sufficient for IDO inhibitory action, and the benzothiazole group was less favorable for inhibiting IDO, because it may restrict the hydrazine–heme interaction.
Based on the co-crystal structure of IDO with PIM, Huang et al.30 discovered that 1H-1,2,3-triazole might be a new key pharmacophore of potent IDO inhibitors. The triazole scaffold shares structural similarity with PIM and it replaces the imidazole ring by a triazole ring, which has been widely applied in pharmaceuticals. The different series of 1,2,3-triazoles were thus synthesized for screening high efficacy IDO inhibitors, and compound 5 showed potent inhibitory activity with an IC50 value of 86 μM. Molecular docking studies indicated that substituent group on 4-aryl should not be too large and an electron-withdrawing group near the NH group of the triazoles was prerequisite of inhibitory activities.
5. IDO inhibitors discovered by HTS
HTS strategy is carried out extensively on library screening by measuring kynurenine formation in enzymatic assay with purified recombinant human IDO protein. The benzylisothiourea derivative 6 was identified as noncompetitive IDO inhibitors via a screening campaign. Subsequent optimization of 6 leads to the identification of the nanomolar inhibitor 7, which suppressed the kynurenine production in A431 cells.31 Structure–activity relationships (SARs) analysis revealed that the distance from the isothiourea moiety and the halogen atom at the C-4 of phenyl ring were necessary for inhibitory activities. The keto-indole derivative 8 was identified as a potent IDO inhibitor via a virtual screening strategy combining various filters, including HTS docking.32 Then, a novel series of IDO inhibitors were synthesized based on this indol-2-yl ethanone scaffold, leading to compound 9 with an IC50 value of 13 μM.33 Preliminary SARs analysis showed that an iron coordinating group on the linker is essential to retain the inhibitory activity. Methylthiohydantoin-tryptophan (MTH-Trp, 10) was discovered by library screening in 2005. A series of novel tryptoline derivatives were synthesized based on its structure and the most active compound 11 showed potent inhibitory activity with an IC50 value of 46.1 μM.34Matsuno et al.35 discovered the anti-hypertensive drug candesartan cilexetil as an IDO inhibitor with an IC50 of 12 μM by screening. This agent is an angiotensin II receptor blocker (ARB) with wide utilities in clinics. A series of candesartan derivatives have subsequently been synthesized and the most active compound 12 showed potent inhibitory activity with an IC50 value of 1.0 μM. SARs analysis and docking results indicated that candesartan derivatives may uniquely bind to the entrance of the active site of IDO, not to the heme ferrous iron. Recently, a series of phenyl benzenesulfonylhydrazides were synthesized to improve cellular inhibitory activities of 2-phenyl benzeneethanesulfonyl-hydrazide 13, which was identified as a potent IDO inhibitor via HTS.36 Compound 14 showed good inhibitory activity with an IC50 value of 61 nM in vitro and an EC50 value of 172 nM in the HeLa cell. SARs analysis revealed that the interactions between 14 and IDO concluded the coordination of sulfone and heme iron, the hydrogen bonding and the hydrophobic interactions.
6. Natural-source IDO inhibitors
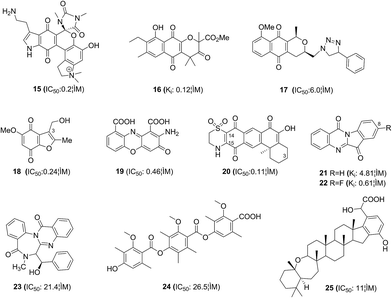
It is well established that various benzoquinone and naphthoquinones display low micromolar activities as IDO inhibitors, e.g. exiguamine A (15) and annulin B (16), suggesting that these moieties are key pharmacophores and likely to be indolemimetic. Bridewell et al.37 tested a series of pyranonaphthoquinone natural products, leading triazole 17 with potent IDO inhibitory activity (IC50: 6.0 μM) and cellular activity against IDO.Benzofuranquinones 18 containing a CH2OR group at C-3 was an analogue of the marine metabolite annulin A.38 It was a potent IDO inhibitor with an IC50 value of 0.24 μM, and this quinone did not generate significant oxidative stress at its IDO inhibitory concentration. Pasceri et al.39 prepared a range of 2-aminophenoxazin-3-ones by the oxidative cyclocondensation of 2-aminophenols. Cinnabarinic acid 19 containing additional electron-withdrawing carboxylate groups on the phenyl rings showed potent inhibitory activity with an IC50 value of 0.46 μM. Meroterpenoid 20 was isolated from the marine sponge Xestospongia vansoesti and showed potent IDO inhibitory activity with an IC50 value of 0.11 μM.40 SARs analysis showed that the orientation of the dioxydihydrothiazine ring fusion to C-14/C-15 is important and the hydroxyl at C-3 is significantly detrimental to the inhibitory activity. The results described above have extended the quinone series with IDO inhibitory activities based on natural products.
Many natural alkaloids have also been discovered to be novel IDO inhibitors. Tryptanthrin(indolo[2,1-b]quinazolin-6,12-dione, 21), a potent IDO inhibitor, was isolated from Chinese medicinal plants Polygonum tinctorium and Isatis tinctoria.41 Three series of tryptanthrin derivatives were synthesized and compound 22 showed potent inhibitory activity with an IC50 value of 0.16 μM. SARs analysis showed that an electron-withdrawing group at the C-8 contributed to IDO inhibition.
In the screening course of the extracts of fungus for IDO inhibitors, a new benzodiazepine alkaloid named benzomalvin E (23) and a new benzoate trimer named thielavin Q (24) were isolated from soil fungus.42,43 They exhibited potent IDO inhibitory activity with IC50 values of 21.4 μM and 26.5 μM, respectively. Williams et al.44 reported new merohexaprenoid, namely, halicloic acid A (25), which was isolated from the marine sponge Haliclona (Halichoclona) sp. collected in the Philippines. This compound exhibited potent IDO inhibitory activity with an IC50 value of 11 μM. SARs analysis indicated that the hexaprenoid moiety is critical to retain its IDO inhibitory activity.
7. Conclusions and perspectives
The structure of IDO consists of a highly conserved sequence AGGSAG (residues 260–265), which provides conformational flexibility in several proteins. Thus, the main chain of IDO exhibits backbone flexibility and a large conformational change, leading to different active site conformations characterized by larger pocket A and different shapes and sizes of pocket B. Therefore, it is important in the approach of IDO docking studies that protein flexibility and heme interactions could be taken into account.IDO is an intercellular monomeric enzyme constituted by a heme Fe iron center as a site for transferring oxygen atoms into pyrrole ring. IDO is active when the Fe iron is in its reduced ferrous state, so the continuous presence of reductants is necessary to maintain the enzyme activity, because IDO is prone to autoxidation. Cytochrome reductases and cytochrome b5 are putative intracellular reducing agents, and these reductants are substituted by a methylene blue and ascorbic acid in vitro assay, to maintain the Fe iron in its reduced form. A large number of natural IDO inhibitors are redox-cycling compounds. Some experts have presumed that these compounds contain problematic functional groups such as quinines, which will not only affect the kynurenine pathway of TRP degradation but also interfere with many other cellular pathways. Therefore, the use these compounds as IDO inhibitors should be avoided (Fig. 5).
IDO is an attractive target for anticancer therapy and the discovery of IDO inhibitors has been intensely ongoing in both academic research laboratories and pharmaceutical organizations. Over the recent five years, several new IDO inhibitor scaffolds have been discovered by structure-based design, HTS, and natural product screening. We hope the information provided herein will be helpful in the development of novel IDO pharmacological inhibitors in the treatment of cancer.
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| ARB | Angiotensin II receptor blocker |
| Bin1 | Bridging integrator 1 |
| CAR-T | Chimeric T cell antigen receptors |
| CTLA-4 | Cytotoxic T lymphocyte antigen-4 |
| DMBA | Dimethylbenzanthracene |
| eIF-2α | Eukaryotic translation initiation factor-2α |
| FDA | Food and drug administration |
| GCN2 | General control non-derepressible 2 |
| GLK1 | Master amino acid-sensing kinase 1 |
| HTS | High throughput screening |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-γ | Interferon-gamma |
| KYN | Kynurenine |
| MM | Metastatic melanoma |
| MTH-Trp | Methylthiohydantoin-tryptophan |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Master metabolic regulator mTOR |
| NF-κB | Nuclear factor-κB |
| NK | Natural killer |
| NSCLC | Non-small-cell lung cancer |
| PD-1 | Programmed cell death-1 |
| PD-L1 | Programmed cell death ligand-1 |
| PDB | Protein data bank |
| PIM | 4-Phenyl-imidazole |
| SARs | Structure–activity relationships |
| STAT1 | Signal transducer and activator of transcription 1 |
| TPA | Terephthalic acid |
| Tregs | T regulatory cells |
| TRP | Tryptophan |
| VCAM-1 | Vascular cell adhesion molecule-1 |
Acknowledgements
The study was supported by the National Natural Science Foundation of China (81302647), the Scientific Research Fund of Sichuan Provincial Education Department (15ZA0132), the Open Research Subject of Key Laboratory of Advanced Scientific Computation and Simulation (SZJJ2014-083) and the innovation fund of post graduate from Xihua University (ycjj2015139).References
- T. Z. Iversen, M. H. Andersen and I. M. Svane, Basic Clin. Pharmacol. Toxicol., 2015, 116, 19–24 CrossRef CAS.
- D. H. Munn, M. D. Sharma, B. Baban, H. P. Harding, Y. H. Zhang, D. Ron and A. L. Mellor, Immunity, 2005, 22, 633–642 CrossRef CAS.
- R. Metz, S. Rust, J. B. DuHadaway, M. R. Mautino, D. H. Munn, N. N. Vahanian, C. J. Link and G. C. Prendergast, Oncoimmunology, 2012, 1, 1460–1468 CrossRef.
- C. A. Opitz, U. M. Litzenburger, F. Sahm, M. Ott, I. Tritschler, S. Trump, T. Schumacher, L. Jestaedt, D. Schrenk, M. Weller, M. Jugold, G. J. Guillemin, C. L. Miller, C. Lutz, B. Radlwimmer, I. Lehmann, A. von Deimling, W. Wick and M. Platten, Nature, 2011, 478, 197–203 CrossRef CAS.
- J. D. Mezrich, J. H. Fechner, X. Zhang, B. P. Johnson, W. J. Burlingham and C. A. Bradfield, J. Immunol., 2010, 185, 3190–3198 CrossRef CAS.
- A. J. Muller, M. D. Sharma, P. R. Chandler, J. B. DuHadaway, M. E. Everhart, B. A. Johnson III, D. J. Kahler, J. Pihkala, A. P. Soler, D. H. Munn, G. C. Prendergast and A. L. Mellor, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17073–17078 CrossRef CAS.
- A. J. Muller, J. B. DuHadaway, M. Y. Chang, A. Ramalingam, E. Sutanto-Ward, J. Boulden, A. P. Soler, L. Mandik-Nayak, S. K. Gilmour and G. C. Prendergast, Cancer Immunol. Immunother., 2010, 59, 1655–1663 CrossRef CAS.
- D. Sakamuro, K. J. Elliott, R. WechslerReya and G. C. Prendergast, Nat. Genet., 1996, 14, 69–77 CrossRef CAS PubMed.
- V. Lindstrom, J. Aittoniemi, J. Jylhava, C. Eklund, M. Hurme, T. Paavonen, S. S. Oja, M. Itala-Remes and M. Sinisalo, Clin. Lymphoma, Myeloma Leuk., 2012, 12, 363–365 CrossRef CAS PubMed.
- Y. Jia, H. Wang, Y. Wang, T. Wang, M. Wang, M. Ma, Y. Duan, X. Meng and L. Liu, Int. J. Cancer, 2015, 137, 1095–1106 CrossRef CAS PubMed.
- A. J. Muller, J. B. DuHadaway, P. S. Donover, E. Sutanto-Ward and G. C. Prendergast, Nat. Med., 2005, 11, 312–319 CrossRef CAS PubMed.
- L. Zitvogel, L. Apetoh, F. Ghiringhelli and G. Kroemer, Nat. Rev. Immunol., 2008, 8, 59–73 CrossRef CAS PubMed.
- K. A. Williams, F. T. Hakim and R. E. Gress, Semin. Immunol., 2007, 19, 318–330 CrossRef CAS PubMed.
- Y. Ma, O. Kepp, F. Ghiringhelli, L. Apetoh, L. Aymerica, C. Locher, A. Tesniere, I. Martins, A. Ly, N. M. Haynes, M. J. Smyth, G. Kroemer and L. Zitvogel, Semin. Immunol., 2010, 22, 113–124 CrossRef CAS PubMed.
- R. B. Holmgaard, D. Zamarin, D. H. Munn, J. D. Wolchok and J. P. Allison, J. Exp. Med., 2013, 210, 1389–1402 CrossRef CAS PubMed.
- S. Spranger, H. K. Koblish, B. Horton, P. A. Scherle, R. Newton and T. F. Gajewski, Journal for immunotherapy of cancer, 2014, 2, 3 CrossRef PubMed.
- U. F. Röhrig, S. R. Majjigapu, P. Vogel, V. Zoete and O. Michielin, J. Med. Chem., 2015, 58, 9421–9437 CrossRef PubMed.
- E. W. Yue, B. Douty, B. Wayland, M. Bower, X. Liu, L. Leffet, Q. Wang, K. J. Bowman, M. J. Hansbury, C. Liu, M. Wei, Y. Li, R. Wynn, T. C. Burn, H. K. Koblish, J. S. Fridman, B. Metcalf, P. A. Scherle and A. P. Combs, J. Med. Chem., 2009, 52, 7364–7367 CrossRef CAS PubMed.
- X. Liu, N. Shin, H. K. Koblish, G. Yang, Q. Wang, K. Wang, L. Leffet, M. J. Hansbury, B. Thomas, M. Rupar, P. Waeltz, K. J. Bowman, P. Polam, R. B. Sparks, E. W. Yue, Y. Li, R. Wynn, J. S. Fridman, T. C. Burn, A. P. Combs, R. C. Newton and P. A. Scherle, Blood, 2010, 115, 3520–3530 CrossRef CAS PubMed.
- H. K. Koblish, M. J. Hansbury, K. J. Bowman, G. Yang, C. L. Neilan, P. J. Haley, T. C. Burn, P. Waeltz, R. B. Sparks, E. W. Yue, A. P. Combs, P. A. Scherle, K. Vaddi and J. S. Fridman, Mol. Cancer Ther., 2010, 9, 489–498 CrossRef CAS PubMed.
- M. R. Mautino, F. A. Jaipun, J. Waldo, S. Kumar, J. Adams, C. Van Allen, A. Marcinowicz-Flick, D. Munn, N. Vahanian and C. J. Link, Cancer Res., 2013, 73, 491 Search PubMed.
- M. Li, A. R. Bolduc, M. N. Hoda, D. N. Gamble, S.-B. Dolisca, A. K. Bolduc, K. Hoang, C. Ashley, D. McCall, A. M. Rojiani, B. L. Maria, O. Rixe, T. J. MacDonald, P. S. Heeger, A. L. Mellor, D. H. Munn and T. S. Johnson, Journal for immunotherapy of cancer, 2014, 2, 21 CrossRef PubMed.
- Z. H. A. Nayak, R. Sadek, R. Dobbins, L. Marshall, N. N. Vahanian, W. J. Ramsey, E. Kennedy, M. Mautino, C. Link, R. Lin, S. Royer-Joo, K. Morrissey, S. Mahrus, B. McCall, A. Pirzkall, J. E. J. D. H. Munn and S. N. Khleif, European Society for Medical Oncology, 2015, 25–29 Search PubMed.
- H. Sugimoto, S. I. Oda, T. Otsuki, T. Hino, T. Yoshida and Y. Shiro, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2611–2616 CrossRef CAS PubMed.
- S. Tojo, T. Kohno, T. Tanaka, S. Kamioka, Y. Ota, T. Ishii, K. Kamimoto, S. Asano and Y. Isobe, ACS Med. Chem. Lett., 2014, 5, 1119–1123 CrossRef CAS PubMed.
- U. F. Röhrig, L. Awad, A. Grosdidier, P. Larrieu, V. Stroobant, D. Colau, V. Cerundolo, A. J. G. Simpson, P. Vogel, B. J. Van den Eynde, V. Zoete and O. Michielin, J. Med. Chem., 2010, 53, 1172–1189 CrossRef PubMed.
- H. Nakashima, K. Ikkyu, K. Nakashima, K. Sano, Y. Uto, E. Nakata, H. Nagasawa, H. Sugimoto, Y. Shiro, Y. Nakagawa and H. Hori, in Oxygen Transport To Tissue Xxxi, ed. E. Takahashi and D. F. Bruley, 2010, vol. 662, pp. 415–421 Search PubMed.
- J. R. Smith, K. J. Evans, A. Wright, R. D. Willows, J. F. Jamie and R. Griffith, Bioorg. Med. Chem., 2012, 20, 1354–1363 CrossRef CAS PubMed.
- S.-P. S. Fung, H. Wang, P. Tomek, C. J. Squire, J. U. Flanagan, B. D. Palmer, D. J. A. Bridewell, S. M. Tijono, J. F. Jamie and L.-M. Ching, Bioorg. Med. Chem., 2013, 21, 7595–7603 CrossRef CAS PubMed.
- Q. Huang, M. Zheng, S. Yang, C. Kuang, C. Yu and Q. Yang, European Journal Of Medicinal Chemistry, 2011, 46, 5680–5687 CrossRef CAS PubMed.
- K. Matsuno, K. Takai, Y. Isaka, Y. Unno, M. Sato, O. Takikawa and A. Asai, Bioorg. Med. Chem. Lett., 2010, 20, 5126–5129 CrossRef CAS PubMed.
- E. Dolusic, P. Larrieu, S. Blanc, F. Sapunaric, J. Pouyez, L. Moineaux, D. Colette, V. Stroobant, L. Pilotte, D. Colau, T. Ferain, G. Fraser, M. Galleni, J.-M. Frere, B. Masereel, B. Van den Eynde, J. Wouters and R. Frederick, European Journal Of Medicinal Chemistry, 2011, 46, 3058–3065 CrossRef CAS PubMed.
- E. Dolusic, P. Larrieu, S. Blanc, F. Sapunaric, B. Norberg, L. Moineaux, D. Colette, V. Stroobant, L. Pilotte, D. Colau, T. Ferain, G. Fraser, M. Galeni, J.-M. Frere, B. Masereel, B. Van den Eynde, J. Wouters and R. Frederick, Bioorg. Med. Chem., 2011, 19, 1550–1561 CrossRef CAS PubMed.
- M. Tanaka, X. Li, H. Hikawa, T. Suzuki, K. Tsutsumi, M. Sato, O. Takikawa, H. Suzuki and Y. Yokoyama, Bioorg. Med. Chem., 2013, 21, 1159–1165 CrossRef CAS PubMed.
- K. Matsuno, H. Yamazaki, Y. Isaka, K. Takai, Y. Unno, N. Ogo, Y. Ishikawa, S. Fujii, O. Takikawa and A. Asai, MedChemComm, 2012, 3, 475–479 RSC.
- M.-F. Cheng, M.-S. Hung, J.-S. Song, S.-Y. Lin, F.-Y. Liao, M.-H. Wu, W. Hsiao, C.-L. Hsieh, J.-S. Wu, Y.-S. Chao, C. Shih, S.-Y. Wu and S.-H. Ueng, Bioorg. Med. Chem. Lett., 2014, 24, 3403–3406 CrossRef CAS PubMed.
- D. J. A. Bridewell, J. Sperry, J. R. Smith, P. Kosim-Satyaputra, L.-M. Ching, J. F. Jamie and M. A. Brimble, Aust. J. Chem., 2013, 66, 40–49 CrossRef CAS.
- C. Carvalho, D. Siegel, M. Inman, R. Xiong, D. Ross and C. J. Moody, Org. Biomol. Chem., 2014, 12, 2663–2674 CAS.
- R. Pasceri, D. Siegel, D. Ross and C. J. Moody, J. Med. Chem., 2013, 56, 3310–3317 CrossRef CAS PubMed.
- R. M. Centko, A. Steino, F. I. Rosell, B. O. Patrick, N. de Voogd, A. G. Mauk and R. J. Andersen, Org. Lett., 2014, 16, 6480–6483 CrossRef CAS PubMed.
- S. Yang, X. Li, F. Hu, Y. Li, Y. Yang, J. Yan, C. Kuang and Q. Yang, J. Med. Chem., 2013, 56, 8321–8331 CrossRef CAS PubMed.
- J.-P. Jang, J.-H. Jang, N.-K. Soung, H.-M. Kim, S.-J. Jeong, Y. Asami, K.-S. Shin, M. R. Kim, H. Oh, B. Y. Kim and J. S. Ahn, J. Antibiot., 2012, 65, 215–217 CrossRef CAS PubMed.
- J.-P. Jang, J.-H. Jang, M. Oh, S. Son, S. M. Kim, H.-M. Kim, K.-S. Shin, H. Oh, N. K. Soung, Y.-S. Hong, B. Y. Kim and J. S. Ahn, J. Antibiot., 2014, 67, 331–333 CrossRef CAS PubMed.
- D. E. Williams, A. Steino, N. J. de Voogd, A. G. Mauk and R. J. Andersen, J. Nat. Prod., 2012, 75, 1451–1458 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |