Linking photoluminescence of α-Si3N4 to intrinsic point defects via band structure modelling
Zhifeng Huanga,
Fei Chen*ab,
Qiang Shena and
Lianmeng Zhanga
aState Key Lab of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China. E-mail: chenfei027@gmail.com; Fax: +86-27-87879468; Tel: +86-27-87168606
bMassachusetts Institute of Technology, 77 Massachusetts Avenue, W20-021 Cambridge, MA 02139-4307, USA
First published on 14th January 2016
Abstract
Photoluminescence (PL) property data for reported α-Si3N4 to date are scattered and it is difficult to clarify the reason only through experimental study. In this paper, the relationship between photoluminescence and intrinsic point defects in α-Si3N4 has been established accordingly via band structure modelling based on density functional theory calculation. The results show that band structures of α-Si3N4 are significantly affected by the locations of defect levels, which are changed with various partial atomic structures around point defects. Formation energies are also calculated to determine the main types of intrinsic point defects in α-Si3N4 under different conditions (Si-rich or N-rich). The results suggest that multiple types of intrinsic point defects coexist in α-Si3N4, leading to various electronic transition modes. Furthermore, combined with the calculated results, the discrete photoluminescence data for α-Si3N4 are revealed by electronic transition modes under different conditions. The main visible luminescent peaks are attributed to the electron transitions of Ec → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ and
Si˙ σ and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ → Ev for Si abundant α-Si3N4, while N4+ → N20 for N plentiful α-Si3N4.
Si˙ σ → Ev for Si abundant α-Si3N4, while N4+ → N20 for N plentiful α-Si3N4.
1. Introduction
Silicon nitride (Si3N4) is a wide band gap (∼5.0 eV) semiconductor material,1,2 which has drawn much attention in the optoelectrical fields for its significant visible luminescence properties due to the wide defect levels.3–5 There are four types of defects in intrinsic point defect Si3N4: N dangling bond (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙), Si dangling bond (
N˙), Si dangling bond (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙), Si–Si and N–N bonds.6–11 The
Si˙), Si–Si and N–N bonds.6–11 The ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙ forms two defect levels in the band gap, namely N20 and N4+, which are near the top of the valence band (VB) and the bottom of the conduction band (CB), respectively.7,8 The
N˙ forms two defect levels in the band gap, namely N20 and N4+, which are near the top of the valence band (VB) and the bottom of the conduction band (CB), respectively.7,8 The ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ forms one defect level at about the middle of the band gap according to previous works.6–9 However, recent work shows the defect levels of
Si˙ forms one defect level at about the middle of the band gap according to previous works.6–9 However, recent work shows the defect levels of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ can be either occupied or unoccupied in the band gap by L. E. Hintzsche.10 The Si–Si bond forms an occupied σ state close or on the top of VB and an empty σ* state close or on the bottom of CB, and the N–N bond gives rise to a filled state in the VB and an empty state in the CB.6–9 Nevertheless, the majority of published studies on related defects have been primarily focused on nonstoichiometric amorphous SiNx films or clusters rather than bulk crystals.6–11
Si˙ can be either occupied or unoccupied in the band gap by L. E. Hintzsche.10 The Si–Si bond forms an occupied σ state close or on the top of VB and an empty σ* state close or on the bottom of CB, and the N–N bond gives rise to a filled state in the VB and an empty state in the CB.6–9 Nevertheless, the majority of published studies on related defects have been primarily focused on nonstoichiometric amorphous SiNx films or clusters rather than bulk crystals.6–11
Density functional theory (DFT) offers an effective method to investigate physical properties for bulk crystals.12,13 A lot of efforts based on DFT have been put on the mechanical, thermodynamic, optical and electronic properties for crystalline Si3N4.14–20 Crystalline Si3N4 has two stable polymorphs at atmospheric environment, i.e. α-Si3N4 (trigonal, space group P31c) and β-Si3N4 (hexagonal, space group P63). α-Si3N4 is dynamically metastable phase under low temperature, and its periodic stacking structure of Si and N atoms is …ABCDABCD…, which is relatively complicated compared to …ABAB… for β-Si3N4.17,18 Defect structures and electronic properties have been reported for intrinsic defects of β-Si3N4,19,20 while little for α-Si3N4. Besides, the types of defects are mostly affected by the growth conditions.21 Herein, to make clear the main point defect types in α-Si3N4 under different conditions, the formation energies for α-Si3N4 with different types of intrinsic point defects under N-rich and Si-rich conditions are calculated in this work.
To date, the photoluminescence properties for crystalline α-Si3N4 are experimentally diverse due to the electron transition mechanisms still unclear.22–25 For instance, the luminescence peak for α-Si3N4 at ∼600 nm (∼2.1 eV) could be explained by electron transition of N4+ → N20 by F. Gao,25 or Ec → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ by Y. Liu.26 Some researchers concluded that the electron transition of Ec →
Si˙ by Y. Liu.26 Some researchers concluded that the electron transition of Ec → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ or
Si˙ or ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ → Ev could form the emission peaks between 2.3 eV and 2.6 eV,8,27 which is in conflict with the points that the
Si˙ → Ev could form the emission peaks between 2.3 eV and 2.6 eV,8,27 which is in conflict with the points that the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ → Ev produce the peaks at about 3.0 eV.28,29 Moreover, the
Si˙ → Ev produce the peaks at about 3.0 eV.28,29 Moreover, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙ would be grabbed by any surplus Si atom, in other words, the
N˙ would be grabbed by any surplus Si atom, in other words, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙ do not coexist with the
N˙ do not coexist with the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙.10 It is controversial to consider the
Si˙.10 It is controversial to consider the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙ and
N˙ and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ simultaneously in the same system to explore the electron transition mechanism.28,29 Therefore, lacking investigations of band structures for intrinsic point defects of α-Si3N4 is the main reason resulting in diverse explanations for electron transition mechanisms.
Si˙ simultaneously in the same system to explore the electron transition mechanism.28,29 Therefore, lacking investigations of band structures for intrinsic point defects of α-Si3N4 is the main reason resulting in diverse explanations for electron transition mechanisms.
In this paper, we devotes to link photoluminescence properties of α-Si3N4 to intrinsic point defects via band structure modelling based on DFT calculation. The intrinsic point defects: Si vacancy (VSi), N vacancy (VN), N substitutional to Si (SiN), Si substitutional to N (NSi), Si interstitial (Sii) and N interstitial (Ni) are systematically considered. Firstly, the geometry optimizations for α-Si3N4 with intrinsic point defects are carried out to study the partial atomic environments near the point defects. Secondly, the electronic properties are calculated with the hybrid Heyd–Scuseria–Ernzerhof (HSE06) functional30,31 to illustrate the influences of different intrinsic point defects on the band structures in α-Si3N4. Thirdly, the formation energies are calculated to determine the main types of intrinsic point defects in α-Si3N4 under the N-rich and Si-rich conditions, respectively. Finally, combined with the calculated results, the electron transitions contributing to the photoluminescence peaks are discussed for Si abundant and N plentiful α-Si3N4.
2. Theoretical and computational methods
The calculations were performed using CASTEP package based on the plane wave pseudo potential approach.32 The optimized structures are obtained with the exchange-correlation functional was Perdew Burke Ernzerhof (PBE) of the generalized gradient approximation (GGA).33 Then, the band structures and density of states were conducted by the HSE06 hybrid functional. To guarantee the calculation accuracy and efficiency, the 2 × 2 × 1 α-Si3N4 supercells with intrinsic point defects were calculated in this paper. A plane-wave cutoff energy of 400 eV and a k-point mesh of 3 × 3 × 5 were used. Reference configurations for the valence electrons were Si 3s 3p and N 2s 2p. The threshold for self-consistent field iterations was 1.0 × 10−5 eV per atom. The convergence tolerance parameters of optimized calculation were the energy of 2 × 10−5 eV per atom, the maximum force of 0.05 eV Å, the maximum inner stress of 0.1 GPa and the maximum displacement of 2 × 10−4 nm.The lattice parameters and the density of states (DOS) of perfect α-Si3N4 supercells were calculated to validate the calculation schemes. The optimized lattice parameters are: a = b = 7.78 Å and c = 5.64 Å, and the calculated energy gap (Eg) by HSE06 hybrid functional is 5.37 eV, which are in good agreement with the experimental value.2 Through analyzing the types of atoms and bond lengths, there are two types of Si atoms (Si1 and Si2) and four types of N atoms (N1, N2, N3 and N4) in α-Si3N4, as shown in Fig. 1. It is seen from Fig. 1 that the Si–N bond lengths of N1 and N2 are the same, respectively. The total and partial DOS of different atoms in perfect α-Si3N4 are plotted in Fig. 2, where the Fermi levels (EF) are set at 0 eV as the dashed lines. It is observed clearly from Fig. 2 that the contributions to the total DOS of the Si1 and Si2 are almost the same, as well as the N3 and N4. Note that the peak on the top of VB for the total DOS is main composed with N pπ states and its width depends on the second-neighbor N–N (pσ) interactions.6 Different to the other types of N atoms, the tailing of the VB for the N1 is significantly beyond the EF resulting from the much larger pσ interactions for its planar configuration. The calculated DOS results are consistent with the previous calculations.6,34 The above calculated results for perfect α-Si3N4 supercells confirm that our calculation schemes are reliable.
 | ||
| Fig. 1 Schematic diagram for different types of atoms in perfect α-Si3N4. The unit of bond length is Å. | ||
Considering there are two different Si atoms and four different N atoms in perfect α-Si3N4, six different vacant and six different substituted α-Si3N4 supercells are taken into account. Besides, there is a big interstice between the AB layer and CD layer in α-Si3N4,17,18 so that two interstitial α-Si3N4 supercells are also considered. α-Si3N4 with vacant defects, one atom (Si or N) removed, are marked as VSi1–Si3N4 and VSi2–Si3N4 for Si vacancy, while VN1–Si3N4, VN2–Si3N4, VN3–Si3N4 and VN4–Si3N4 for N vacancy. α-Si3N4 with substitutional defects, one atom (Si or N) in the forms of a substitutional atom (SiN or NSi), are marked as SiN1–Si3N4, SiN2–Si3N4, SiN3–Si3N4 and SiN4–Si3N4 for Si substituting to N, while NSi1–Si3N4 and NSi2–Si3N4 for N substituting to Si. α-Si3N4 with interstitial defects, one atom (Si or N) occupying the interstice, are marked as Sii–Si3N4 and Ni–Si3N4, respectively. After finishing geometry optimizations of all α-Si3N4 supercells with intrinsic point defects by the functional of GGA, the electronic properties are calculated with the HSE06 hybrid functional.
3. Results and discussion
3.1 Optimal crystal structures of α-Si3N4 with intrinsic point defects
Table 1 shows the lattice parameters and lengths of the new bonds for the optimal α-Si3N4 supercells with different intrinsic point defects. The variations of lattice parameters for α-Si3N4 with intrinsic point defects come from the changes of the partial atomic environments near the point defects. The partial atomic structures near the investigated point defects in α-Si3N4 are presented in Fig. 3. For α-Si3N4 with Si vacant defect (shown in Fig. 3(a) and (b)), the distances become larger between each two N atoms compared with that of ∼2.80 Å in perfect α-Si3N4, and no new bond is formed around the Si vacancy, leaving four unsaturated N dangling bonds (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙). For α-Si3N4 with N vacant defect, the three nearest Si atoms are not equivalent,16 with slightly shortening the distances of them (the distance of two nearest Si atoms is 2.96 Å in perfect α-Si3N4), to form two Si–Si bonds in the VN1–Si3N4 and VN2–Si3N4 (shown in Fig. 3(c) and (d)), one Si–Si bond in the VN3–Si3N4 and VN4–Si3N4 (shown in Fig. 3(e) and (f)), respectively. For α-Si3N4 with Si substituting to N defect (shown in Fig. 3(g)–(j)), the substitutional Si atom forms three Si–Si bonds with its ambient Si atoms, and the extra electron of the substitutional Si atom forms one Si dangling bond (
N˙). For α-Si3N4 with N vacant defect, the three nearest Si atoms are not equivalent,16 with slightly shortening the distances of them (the distance of two nearest Si atoms is 2.96 Å in perfect α-Si3N4), to form two Si–Si bonds in the VN1–Si3N4 and VN2–Si3N4 (shown in Fig. 3(c) and (d)), one Si–Si bond in the VN3–Si3N4 and VN4–Si3N4 (shown in Fig. 3(e) and (f)), respectively. For α-Si3N4 with Si substituting to N defect (shown in Fig. 3(g)–(j)), the substitutional Si atom forms three Si–Si bonds with its ambient Si atoms, and the extra electron of the substitutional Si atom forms one Si dangling bond (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙).19 It could be observed that the lengths of the three Si–Si bonds are the same in the SiN1–Si3N4 and SiN2–Si3N4 (shown in Fig. 3(g) and (h)) while different in the SiN3–Si3N4 and SiN4–Si3N4 (shown in Fig. 3(i) and (j)), attributed to different coordination environments of different types of N atoms, as shown in Fig. 1. For α-Si3N4 with N substituting to Si defect, the substitutional N atom forms three N–N bonds with its adjacent N atoms and leaves an unsaturated matrix N atom to form one N dangling bond (shown in Fig. 3(k) and (l)). For α-Si3N4 with Si interstitial defect (shown in Fig. 3(m)), the interstitial Si atom forms two strong Si–Si bond and three weak Si–N bonds with the matrix atoms.20 For α-Si3N4 with interstitial N defect, the unsaturated interstitial N atom forms one N–N bond and one new Si–N bond with the matrix Si and N atoms (shown in Fig. 3(n)). Compared with the optimized partial structures of all defective models, the VSi1–Si3N4 and VSi2–Si3N4, as well as the VN3–Si3N4 and VN4–Si3N4, the SiN3–Si3N4 and SiN4–Si3N4, as well as the NSi1–Si3N4 and NSi2–Si3N4 are almost the same. The main types of defects in α-Si3N4 with different intrinsic point defects are clearly illustrated by the partial atomic structures, whereas the electronic properties associated with the defects are discussed in the next section.
Si˙).19 It could be observed that the lengths of the three Si–Si bonds are the same in the SiN1–Si3N4 and SiN2–Si3N4 (shown in Fig. 3(g) and (h)) while different in the SiN3–Si3N4 and SiN4–Si3N4 (shown in Fig. 3(i) and (j)), attributed to different coordination environments of different types of N atoms, as shown in Fig. 1. For α-Si3N4 with N substituting to Si defect, the substitutional N atom forms three N–N bonds with its adjacent N atoms and leaves an unsaturated matrix N atom to form one N dangling bond (shown in Fig. 3(k) and (l)). For α-Si3N4 with Si interstitial defect (shown in Fig. 3(m)), the interstitial Si atom forms two strong Si–Si bond and three weak Si–N bonds with the matrix atoms.20 For α-Si3N4 with interstitial N defect, the unsaturated interstitial N atom forms one N–N bond and one new Si–N bond with the matrix Si and N atoms (shown in Fig. 3(n)). Compared with the optimized partial structures of all defective models, the VSi1–Si3N4 and VSi2–Si3N4, as well as the VN3–Si3N4 and VN4–Si3N4, the SiN3–Si3N4 and SiN4–Si3N4, as well as the NSi1–Si3N4 and NSi2–Si3N4 are almost the same. The main types of defects in α-Si3N4 with different intrinsic point defects are clearly illustrated by the partial atomic structures, whereas the electronic properties associated with the defects are discussed in the next section.
| Point defect | Cell parameter (Å) | Length of new bond (Å) | ||||
|---|---|---|---|---|---|---|
| a | b | c | Si–N | Si–Si | N–N | |
| a The numbers in the parentheses show the number of bonds with same length. | ||||||
| VSi1 | 7.83 | 7.83 | 5.63 | |||
| VSi2 | 7.84 | 7.78 | 5.63 | |||
| VN1 | 7.75 | 7.78 | 5.63 | 2.70 | ||
| 2.76 | ||||||
| VN2 | 7.75 | 7.78 | 5.64 | 2.58 | ||
| 2.70 | ||||||
| VN3 | 7.79 | 7.76 | 5.65 | 2.56 | ||
| VN4 | 7.76 | 7.78 | 5.65 | 2.58 | ||
| SiN1 | 7.86 | 7.86 | 5.62 | 2.16(3)a | ||
| SiN2 | 7.82 | 7.82 | 5.43 | 2.21(3)a | ||
| SiN3 | 7.79 | 7.82 | 5.66 | 2.15 | ||
| 2.19 | ||||||
| 2.21 | ||||||
| SiN4 | 7.85 | 7.84 | 5.67 | 2.10 | ||
| 2.11 | ||||||
| 2.12 | ||||||
| NSi1 | 7.78 | 7.74 | 5.65 | 1.41 | ||
| 1.45(2)a | ||||||
| NSi2 | 7.77 | 7.79 | 5.65 | 1.42 | ||
| 1.45(2)a | ||||||
| Sii | 7.79 | 7.80 | 5.64 | 2.10 | 2.32 | |
| 2.21 | 2.56 | |||||
| 2.36 | ||||||
| Ni | 7.79 | 7.81 | 5.65 | 1.81 | 1.59 | |
3.2 Band structures of α-Si3N4 with intrinsic point defects
The calculated band structures and partial density of states (PDOS) of the atoms adjacent to the investigated point defects for all defective α-Si3N4 are plotted in Fig. 4, where the Fermi levels (EF) are set at 0 eV as the red dashed lines. It is clear that the band structures and PDOS of the VSi1–Si3N4 and VSi2–Si3N4 (shown in Fig. 4(a) and (b)), the VN3–Si3N4 and VN4–Si3N4 (shown in Fig. 4(e) and (f)), the SiN3–Si3N4 and SiN4–Si3N4 (shown in Fig. 4(i) and (j)), as well as the NSi1–Si3N4 and NSi2–Si3N4 (shown in Fig. 4(k) and (l)) are similar, respectively. Compared with the Eg = 5.37 eV for perfect α-Si3N4, the maximum energy gaps for all intrinsic defective α-Si3N4 become smaller owing to the emergence of defect levels in the band gaps. The Ev and Ec stand for the energies of the top of VB and the bottom of CB, respectively. The energies of N 2s and 2p states appear in the Ev and just below the Ec in the VSi1–Si3N4 and VSi2–Si3N4, respectively, which are in good agreement with the results of Robertson's research,6,7 indicating that N20 and N4+ are the main defects in α-Si3N4 with Si vacant defect. For α-Si3N4 with N vacant defect, the defect levels located in the Ev and below the Ec are related to the Si–Si bonding σ states and antibonding σ* states,6–9 and the else two defect levels in the band gaps are the Si dangling bonds occupied (N3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ) and unoccupied (N3
Si˙ σ) and unoccupied (N3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ*) states.10 Particularly, for the VN1–Si3N4 (see Fig. 4(c)), owing to the lower energy of the Si–Si σ compared with the pσ state, the defect level of the Si–Si σ state is certainly lower than the Ev. Besides, for the VN2–Si3N4 (see Fig. 4(d)), because the short distances between the three Si atoms adjacent to the N vacancy, the unsaturated Si dangling bond are not entirely separated from the Si–Si bonds, causing the overlapping of the N3
Si˙ σ*) states.10 Particularly, for the VN1–Si3N4 (see Fig. 4(c)), owing to the lower energy of the Si–Si σ compared with the pσ state, the defect level of the Si–Si σ state is certainly lower than the Ev. Besides, for the VN2–Si3N4 (see Fig. 4(d)), because the short distances between the three Si atoms adjacent to the N vacancy, the unsaturated Si dangling bond are not entirely separated from the Si–Si bonds, causing the overlapping of the N3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ* and Si–Si σ* states. The distortion of the local Si environment and the Si–Si bond length could influence the location and composition of the electronic states of Si defect in the band gap.10 Unlike the long lengths of Si–Si bonds in α-Si3N4 with N vacancy defect, the Si–Si bonds are particularly short and the three Si–Si bonds are closely around the unsaturated Si dangling band (Si3
Si˙ σ* and Si–Si σ* states. The distortion of the local Si environment and the Si–Si bond length could influence the location and composition of the electronic states of Si defect in the band gap.10 Unlike the long lengths of Si–Si bonds in α-Si3N4 with N vacancy defect, the Si–Si bonds are particularly short and the three Si–Si bonds are closely around the unsaturated Si dangling band (Si3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙) in α-Si3N4 with Si substituting to N defect, resulting in the difference in the locations and compositions of Si–Si bonds and Si3
Si˙) in α-Si3N4 with Si substituting to N defect, resulting in the difference in the locations and compositions of Si–Si bonds and Si3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙. Thus, for α-Si3N4 with Si substituting to N defect, the defect levels close or in the Ev and the Ec are corresponding to the Si–Si σ and σ* states, respectively, and the levels near the EF are the Si3
Si˙. Thus, for α-Si3N4 with Si substituting to N defect, the defect levels close or in the Ev and the Ec are corresponding to the Si–Si σ and σ* states, respectively, and the levels near the EF are the Si3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙. As reported in Robertson's research, the N–N bond is mainly consisted of N 2p states and appearing in the VB or CB.6 So the defect levels in the Ev are the N–N states, and the two defect levels in the band gap are the N20 and N4+ for α-Si3N4 with N substituting to Si defect. Similarly, for the Ni–Si3N4 (shown in Fig. 4(n)), the defect levels composed of N 2p states in the VB and CB are the N–N σ and σ* states, and the defect level near the EF is the unsaturated N dangling bond state. For the Sii–Si3N4 (shown in Fig. 5(m)), the defect levels are consisted with N 2p and Si 3s 3p states in the VB and close to the Ec, which are related to the new Si–N bonds,20 and the defect level near the EF is the unsaturated Si dangling bond.
Si˙. As reported in Robertson's research, the N–N bond is mainly consisted of N 2p states and appearing in the VB or CB.6 So the defect levels in the Ev are the N–N states, and the two defect levels in the band gap are the N20 and N4+ for α-Si3N4 with N substituting to Si defect. Similarly, for the Ni–Si3N4 (shown in Fig. 4(n)), the defect levels composed of N 2p states in the VB and CB are the N–N σ and σ* states, and the defect level near the EF is the unsaturated N dangling bond state. For the Sii–Si3N4 (shown in Fig. 5(m)), the defect levels are consisted with N 2p and Si 3s 3p states in the VB and close to the Ec, which are related to the new Si–N bonds,20 and the defect level near the EF is the unsaturated Si dangling bond.
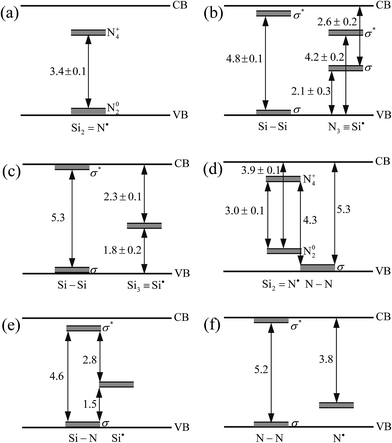 | ||
| Fig. 5 Defect levels and electron transition diagrams in α-Si3N4 with intrinsic point defects: (a) VSi, (b) VN, (c) SiN, (d) NSi, (e) Sii and (f) Ni. The unit of energy gap is eV. | ||
Considering the similar electronic properties for α-Si3N4 with similar intrinsic point defects, Fig. 5 summarizes the defect levels in the band gaps and electron transition diagrams for α-Si3N4 with six main point defects, which are the Si vacant defect (VSi), N vacant defect (VN), Si substituting to N defect (SiN), N substituting to Si defect (NSi), interstitial Si defect (Sii) and interstitial N defect (Ni). The similar types of defect levels in the band gaps are different for the six main point defective models. For example, it is observed from Fig. 5(a) and (d) that the maximum energy gaps of the two N dangling bonds states are ∼3.4 eV in the VSi–Si3N4, while ∼3.0 eV in the NSi–Si3N4. The relative locations of the defect levels of the two Si–Si states integrally move up in the band gaps from the VN–Si3N4 to SiN–Si3N4, as shown in Fig. 5(b) and (c). For the defect levels of the unsaturated Si dangling bonds, there are two levels in the band gap for the VN–Si3N4 (shown in Fig. 5(b)), while only one level located at about the EF in the SiN–Si3N4 and Sii–Si3N4 (shown in Fig. 5(c) and (e)), respectively. The variations for the relative locations of similar defect levels come from different partial atomic environments.10 For instance, the nearby atoms of the unsaturated Si atoms are N atoms in the VN–Si3N4, Si atoms in the SiN–Si3N4, N and Si atoms in the Sii-Si3N4, so that the locations of defect levels originated from the unsaturated Si dangling bands are different for α-Si3N4 with different intrinsic point defects. To further investigate the main defect types under different conditions, the formation energies of α-Si3N4 with different intrinsic point defects are discussed in the next section.
3.3 Formation energy of α-Si3N4 with intrinsic point defects
The stability of different intrinsic point defect α-Si3N4 can be determined by the value of formation energy (Ef(q)), which is obtained by the following formula:20,21,35| Ef(q) = Etot(q) − nSiμSi − nNμN − qEF |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙),10 the N substituting to Si defect and the interstitial N defect do not coexist with the N vacant defect. To make it easy to understand, we define the cases that the atomic ratio of Si and N in α-Si3N4 is more than 3
Si˙),10 the N substituting to Si defect and the interstitial N defect do not coexist with the N vacant defect. To make it easy to understand, we define the cases that the atomic ratio of Si and N in α-Si3N4 is more than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as Si3N4−x, which includes VN–Si3N4, SiN–Si3N4 and Sii–Si3N4. The other cases of Si
4 as Si3N4−x, which includes VN–Si3N4, SiN–Si3N4 and Sii–Si3N4. The other cases of Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N < 3
N < 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 are defined as Si3−xN4, which are NSi–Si3N4, Ni–Si3N4 and VSi–Si3N4. It is clear that Si3N4−x can be easily formed under Si-rich condition, while Si3−xN4 can be prepared under N-rich condition.
4 are defined as Si3−xN4, which are NSi–Si3N4, Ni–Si3N4 and VSi–Si3N4. It is clear that Si3N4−x can be easily formed under Si-rich condition, while Si3−xN4 can be prepared under N-rich condition.
| Point defect | Si-rich | N-rich | Point defect | Si-rich | N-rich |
|---|---|---|---|---|---|
| VSi1 | 11.02 | 7.69 | SiN2 | 4.61 | 10.47 |
| VSi2 | 11.04 | 7.71 | SiN3 | 5.25 | 11.12 |
| VN1 | 3.36 | 5.89 | SiN4 | 5.58 | 11.45 |
| VN2 | 3.37 | 5.90 | NSi1 | 11.60 | 5.76 |
| VN3 | 3.00 | 5.52 | NSi2 | 11.22 | 5.39 |
| VN4 | 3.32 | 5.85 | Sii | 6.15 | 9.50 |
| SiN1 | 4.88 | 10.75 | Ni | 8.23 | 5.74 |
3.4 Linking photoluminescence of α-Si3N4 to intrinsic point defects
The currently reported photoluminescence data for α-Si3N4 are plotted Fig. 6(a).22–24,37–42 It is observed that the PL data is quite scattered, and PL properties are analyzed using different electron transition modes. The reason now is clear because there are multiple intrinsic point defects for Si3N4−x and Si3−xN4, determined by different experiment processing methods, resulting in different electron transition in the band gap, and correspondingly a number of visible luminescence peaks. Some of the broaden peaks may be the integral of several single peak, which reflects certain electron transition mode. According to previous analysis, it is known that for Si3N4−x, the Ev and Ec may be composed of the occupied and empty states of Si–Si or Si–N bond, as exhibited in Fig. 5(b), (c) and (e), respectively. The defects levels in the band gap are related to the unsaturated Si dangling bonds (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙), which may be in different forms as N3
Si˙), which may be in different forms as N3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙, Si3
Si˙, Si3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ or the other unsaturated Si states. On the other hand, for Si3−xN4, the Ev and Ec may be composed of the N–N occupied and empty states as exhibited in Fig. 5(a), (d) and (f). The defect levels in the band gap are the unsaturated N dangling bonds (
Si˙ or the other unsaturated Si states. On the other hand, for Si3−xN4, the Ev and Ec may be composed of the N–N occupied and empty states as exhibited in Fig. 5(a), (d) and (f). The defect levels in the band gap are the unsaturated N dangling bonds (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙).
N˙).
 | ||
| Fig. 6 (a) Photoluminescence data for α-Si3N4 in different literatures. (b) Wavelength ranges of the emission peaks for Si3N4−x (red bar) and Si3−xN4 (blue bars), respectively. | ||
The wavelength ranges of the emission peaks are shown as the red and blue bars in Fig. 6(b) for Si3N4−x and Si3−xN4, respectively. Thus, it is clear that in the Si abundant α-Si3N4, the PL peaks at >440 nm (the corresponding energy gaps < 2.8 eV) are related to the electron transitions of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ → Ev, or Ec →
Si˙ σ → Ev, or Ec → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ. While in the N plentiful α-Si3N4, the PL peaks of 400–430 nm (2.8–3.0 eV) may be driven from the electron transitions of N4+ → N20, and the peaks at <380 nm (>3.3 eV) relate to N4+ → Ev in Si vacant α-Si3N4. For example, the main PL peaks located at >500 nm (ref. 37–39) may be derived from the electron transitions of
Si˙ σ. While in the N plentiful α-Si3N4, the PL peaks of 400–430 nm (2.8–3.0 eV) may be driven from the electron transitions of N4+ → N20, and the peaks at <380 nm (>3.3 eV) relate to N4+ → Ev in Si vacant α-Si3N4. For example, the main PL peaks located at >500 nm (ref. 37–39) may be derived from the electron transitions of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ → Ev or Ec →
Si˙ σ → Ev or Ec → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ, however, the main peaks at 410–430 nm (ref. 23, 24 and 40–42) are related to the electron transitions of N4+ → N20. The current investigation has successfully demonstrate the reason for the discreteness of the PL spectra for α-Si3N4.
Si˙ σ, however, the main peaks at 410–430 nm (ref. 23, 24 and 40–42) are related to the electron transitions of N4+ → N20. The current investigation has successfully demonstrate the reason for the discreteness of the PL spectra for α-Si3N4.
4. Conclusions
Photoluminescence properties of α-Si3N4 have been connected to intrinsic point defects by band structure modelling using DFT calculations. The partial atomic structures, electronic properties and formation energies for α-Si3N4 with different intrinsic point defects are systematically investigated. The Si dangling bonds give rise to two defect levels (N3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ and σ*) in the band gap for the VN–Si3N4, while only one defect level for the SiN–Si3N4 and Sii–Si3N4. The maximum energy gaps between N20 and N4+ are different in the VSi–Si3N4 and NSi–Si3N4. The different locations of the similar defect levels are originated from the various partial atomic environments adjacent to the investigated point defects. Multiple types of intrinsic point defects would coexist in α-Si3N4 under different environments derived from formation energy calculation. The main visible luminescent peaks for α-Si3N4 are attributed to the electron transitions of Ec →
Si˙ σ and σ*) in the band gap for the VN–Si3N4, while only one defect level for the SiN–Si3N4 and Sii–Si3N4. The maximum energy gaps between N20 and N4+ are different in the VSi–Si3N4 and NSi–Si3N4. The different locations of the similar defect levels are originated from the various partial atomic environments adjacent to the investigated point defects. Multiple types of intrinsic point defects would coexist in α-Si3N4 under different environments derived from formation energy calculation. The main visible luminescent peaks for α-Si3N4 are attributed to the electron transitions of Ec → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ and
Si˙ σ and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si˙ σ → Ev for Si3N4−x, while N4+ → N20 for Si3−xN4. This work not only reveals the discrete photoluminescence data for α-Si3N4, but also provides theoretical foundations for electronic properties of Si3N4.
Si˙ σ → Ev for Si3N4−x, while N4+ → N20 for Si3−xN4. This work not only reveals the discrete photoluminescence data for α-Si3N4, but also provides theoretical foundations for electronic properties of Si3N4.
Acknowledgements
The project is supported by the National Natural Science Foundation of China (No. 51202171, No. 51472188, No. 51521001), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120143120004) and the “111” project (No. B13035).Notes and references
- K. Ulman, R. Sathiyanarayanan, R. K. Pandey, K. V. R. M. Murali and S. Narasimhan, J. Appl. Phys., 2013, 113, 234102 CrossRef.
- L. Zhang, H. Jin, W. Yang, Z. Xie, H. Miao and L. An, Appl. Phys. Lett., 2005, 86, 1908 Search PubMed.
- R. J. Xie and N. Hirosaki, Sci. Technol. Adv. Mater., 2007, 8, 588–600 CrossRef CAS.
- J. Cai, Y. L. Zhang, Z. Y. Lyu, J. Zhao, J. C. Shen, Q. Wu and Z. Hu, CrystEngComm, 2015, 17, 23–26 RSC.
- B. Sain and D. Das, Phys. Chem. Chem. Phys., 2013, 15, 3881–3888 RSC.
- J. Robertson, Philos. Mag. B, 1991, 63, 47–77 CAS.
- J. Robertson, Mater. Res. Soc. Symp. Proc., 1993, 284, 65–76 CrossRef CAS.
- S. V. Deshpande, E. Gulari, S. W. Brown and S. C. Rand, J. Appl. Phys., 1995, 77, 6534–6541 CrossRef CAS.
- J. Robertson, W. L. Warren and J. Kanicki, J. Non-Cryst. Solids, 1995, 187, 297–300 CrossRef CAS.
- L. E. Hintzsche, C. M. Fang, M. Marsman, G. Jordan, M. W. P. E. Lamers, A. W. Weeber and G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 155204 CrossRef.
- P. A. Pundur, J. G. Shavalgin and V. A. Gritsenko, Phys. Status Solidi A, 1986, 94, K107–K112 CrossRef CAS.
- S. Baroni, S. De Gironcoli, A. Dal Corso and P. Giannozzi, Rev. Mod. Phys., 2001, 73, 515 CrossRef CAS.
- J. Yu, P. Zhou and Q. Li, Phys. Chem. Chem. Phys., 2013, 15, 12040–12047 RSC.
- D. Legut, U. D. Wdowik and P. Kurtyka, Mater. Chem. Phys., 2014, 147, 42–49 CrossRef CAS.
- G. Kresse, M. Marsman, L. E. Hintzsche and E. Flage-Larsen, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 045205 CrossRef.
- V. A. Gritsenko, S. S. Nekrashevich, V. V. Vasilev and A. V. Shaposhnikov, Microelectron. Eng., 2009, 86, 1866–1869 CrossRef CAS.
- A. Kuwabara, K. Matsunaga and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 064104 CrossRef.
- D. Hardie and K. H. Jack, Nature, 1957, 180, 332 CrossRef CAS.
- C. Di Valentin, G. Palma and G. Pacchioni, J. Phys. Chem. C, 2010, 115, 561–569 Search PubMed.
- M. E. Grillo, S. D. Elliott and C. Freysoldt, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 085208 CrossRef.
- F. Oba, K. Tatsumi, I. Tanaka and H. Adachi, J. Am. Ceram. Soc., 2002, 85, 97–100 CrossRef CAS.
- Z. Wu, Z. Zhang, J. Yun, J. Yan and T. You, Phys. B Condens. Matter, 2013, 428, 10–13 CrossRef CAS.
- L. W. Lin and Y. H. He, CrystEngComm, 2012, 14, 3250–3256 RSC.
- J. Huang, S. Zhang, Z. Huang, Y. Wen, M. Fang and Y. Liu, CrystEngComm, 2012, 14, 7301–7305 RSC.
- F. Gao, W. Yang, Y. Fan and L. An, Nanotechnology, 2008, 19, 105602 CrossRef.
- Y. Liu, Y. Zhou, W. Shi, L. Zhao, B. Sun and T. Ye, Mater. Lett., 2004, 58, 2397–2400 CrossRef CAS.
- G. Zou, B. Hu, K. Xiong, H. Li, C. Dong, J. Liang and Y. Qian, Appl. Phys. Lett., 2005, 86, 1901 Search PubMed.
- H. Du and A. Gao, Mater. Lett., 2012, 84, 31–33 CrossRef CAS.
- Y. Xu, C. Cao, Z. Chen, J. Li, F. Wang and H. Cai, J. Phys. Chem. B, 2006, 110, 3088–3092 CrossRef CAS PubMed.
- A. V. Krukau, O. A. Vydrov, A. F. Izmaylov and G. E. Scuseria, J. Chem. Phys., 2006, 125, 224106 CrossRef PubMed.
- J. Paier, M. Marsman, K. Hummer, G. Kresse, I. C. Gerber and J. G. Ángyán, J. Chem. Phys., 2006, 124, 154709 CrossRef CAS.
- M. C. Payne, M. P. Teter, D. C. Allan, T. A. Arias and J. D. Joannopoulos, Rev. Mod. Phys., 1992, 64, 1045 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- Y. N. Xu and W. Y. Ching, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 17379 CrossRef CAS.
- Y. X. Han, C. L. Yang, M. S. Wang and X. G. Ma, RSC Adv., 2014, 4, 55452–55458 RSC.
- J. Robertson and M. J. Powell, Appl. Phys. Lett., 1984, 44, 415–417 CrossRef CAS.
- M. Ahmad, J. Zhao, C. Pan and J. Zhu, J. Cryst. Growth, 2009, 311, 4486–4490 CrossRef CAS.
- G. Zou, B. Hu, K. Xiong, H. Li, C. Dong, J. Liang and Y. Qian, Appl. Phys. Lett., 2005, 86, 1901 Search PubMed.
- L. W. Yin, Y. Bando, Y. C. Zhu and Y. B. Li, Appl. Phys. Lett., 2003, 83, 3584 CrossRef CAS.
- Y. Xu, C. Cao, H. Du, J. Li and H. Zhu, Mater. Lett., 2007, 61, 3855–3858 CrossRef CAS.
- Y. Xu, C. Cao, Z. Chen, J. Li, F. Wang and H. Cai, J. Phys. Chem. B, 2006, 110, 3088–3092 CrossRef CAS PubMed.
- H. Du and A. Gao, Mater. Lett., 2012, 84, 31–33 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



