Scalable simultaneous activation and separation of metal–organic frameworks†
Abstract
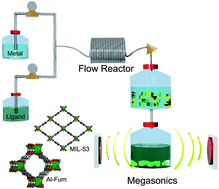
Despite the many promising applications of Metal–Organic Frameworks (MOFs) the key advances to boost production to industrial scale still remain elusive. Recently, it has been shown that continuous flow chemistry is capable of handling the production of a large variety of MOFs, while also being scalable. However commonly used laboratory methods for postproduction processing, i.e. separation and activation are ineffective and costly at scale. Here we present the use of megasonics, a novel and scalable methodology based on sonication with high frequency sound waves, which performs separation and activation simultaneously, yielding high surface areas that previously were only obtainable with laboratory-scale methods like solvent exchange, supercritrics and calcination.

 Please wait while we load your content...
Please wait while we load your content...