In vivo detection of fluoride at trace levels and its removal from raw water at neutral pH utilizing a cyanobacterium pigment as a luminescent probe†
Abstract
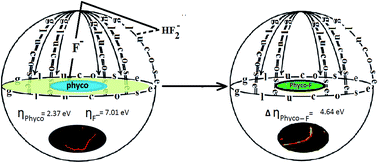
A selective method has been developed for trace level (0.01 mg L−1) fluoride detection in HEPES buffer in the presence of interfering ions (such as Cl−, I−, Br−, SO42−, ClO4−, and CH3COO−) using a cyanobacterium as a luminescent probe. It is able to detect trace levels well below the PHS recommended levels for drinking water (0.7–1.2 ppm), which places this probe among the most sensitive fluoride sensors reported to date. The cellular pigment, phycocyanobilin 2, of living algae plays an anchoring role to sense fluoride at trace levels via instantaneous fluorescence. Algal biomass was used for the removal of fluoride from raw water at neutral pH. The maximum uptake capacity (BTC: 6.76 mg g−1 and Q0: 6.08 mg g−1) and preconcentration factor (PF: 64.2) were found to be appreciably high. Interference caused by the presence of several co-existing ions is also discussed. The proposed method has been applied to real samples, such as pond water, well water and ground water, with good analytical reliability: removal of fluoride up to 92.3% ± 1.3%, with a relative standard deviation of 2–3%, and re-usability of 70–90 cycles.

 Please wait while we load your content...
Please wait while we load your content...