Synthesis of nitrate ester and nitramine derivatives of polyfluoro alkyl compounds for high energy materials†
Abstract
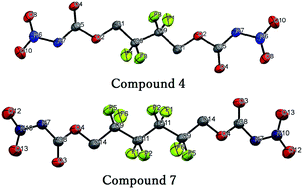
Five new poly-fluorine containing molecules were synthesized from economical and commercially available materials for high energy material applications by straightforward routes. All the synthesized molecules were characterized using IR, NMR spectroscopy, elemental analysis, single crystal X-ray diffraction and thermal (TG-DTA), impact, and friction stability measurements. In addition, the crystal structures of 4, 7 and 8 are presented. The synthesized poly-fluorine compounds were found to have reasonable detonation properties based on calculations (EXPLO5 v6.01), which are in the range of TNT and RDX. These compounds are thermally stable (Tdec > 150 °C) and are insensitive toward impact and friction.

 Please wait while we load your content...
Please wait while we load your content...