Band modification of graphene by using slow Cs+ ions†
Abstract
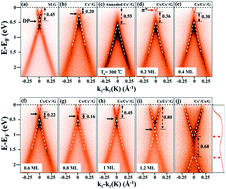
We report new wide band gap engineering for graphene using slow Cs+ ions, which allows both fine-tuning and on–off switching capability of the band gap in a range suitable for most applications without modifying or deteriorating the relativistic nature of the Dirac fermions. The doping of Cs+ ions opens the band gap up to Eg = 0.68 eV, which can be closed completely by adding neutral Cs atoms, as observed in angle-resolved photoemission spectroscopy. The operating mechanism of this band gap engineering is understood by a simple capacitor model, which is fully supported by the density-functional theory calculations.

 Please wait while we load your content...
Please wait while we load your content...