Synthesis and electrochemical study of sodium ion transport polymer gel electrolytes†
Abstract
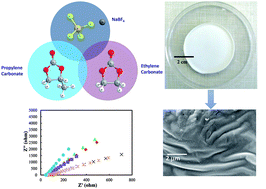
This research demonstrates a new, high conductivity sodium ion polymer gel electrolyte (PGE), which is prepared using a solution casting technique. The prepared PGE consists of a plasticized polymer blend of poly(methyl methacrylate) (PMMA) and polycarbonate that serves as a framework to immobilize phase separated interconnected liquid rich regions of ethylene carbonate (EC) and propylene carbonate (PC). Portions of these liquids that remain dissolved in the polymer blend act as plasticizers while interconnected liquid regions provide an all-liquid conductive pathway. A loosely bonded sodium salt, sodium tetrafluoroborate (NaBF4), was added to the PGE to decrease the crystallinity of the polymer blend, thus lowering energy barriers for ion transfer in the blend and providing more charge carriers in the liquid rich phases to enhance the overall ionic conductivity of the PGE. Peak ionic conductivity of 5.67 × 10−4 S cm−1 was observed from electrochemical impedance spectroscopy measurements of a PGE with 25 wt% NaBF4 which is more than two orders of magnitude larger than the same PGE without NaBF4 that demonstrates a conductivity of 1.03 × 10−6 S cm−1. The temperature dependence of ionic conductivity agrees with the Arrhenius equation from 20 °C to 90 °C. The activation energies for PGEs with different concentrations of NaBF4: 5 wt%, 15 wt% and 25 wt% are found to be 0.13, 0.17 and 0.28 eV respectively. Cyclic voltammetry confirmed that the PGEs are electrochemically stable over a wide potential range of −5 V to +5 V. In addition, transference number measurements, whose values varied from 0.83 to 0.93, demonstrate that these PGEs are ionic conductive electrolytes. The findings of this study are consistent with the development of a sodium ion conductive electrolyte films that are promising for use in non-aqueous advanced energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...