Global spectral and local molecular connects for optical coherence tomography features to classify oral lesions towards unravelling quantitative imaging biomarkers†
Abstract
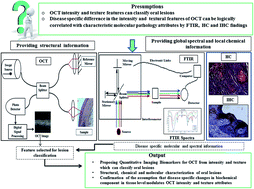
The biopsy based diagnosis of oral precancers like leukoplakia (OLK) and submucous fibrosis (OSF) as well as squamous cell carcinoma (OSCC) suffers from observer specific variability. The present work explores the utility of intensity and textural features from optical coherence tomography (OCT) images after specific feature subset selection for precise classification of oral lesions using variants of support vector machine. Concomitant application of Fourier transform infrared (FTIR) spectroscopy for endorsing global biochemical signatures, and histochemistry was performed further for value addition of the OCT findings. Immunohistochemical findings for characterization of specific local molecular alteration were also included in this. Result suggested that, OCT features could differentiate the lesions with high sensitivity and specificity. The FTIR result showed glycogen, keratin and carbohydrate related alteration in OSCC, decrease in collagen specific amino acids and skeletal muscle related proteins in OSF and distinct variation in tissue hydration status in diseases. There was also increase in keratin layer thickness in OLK due to overexpression of cytokeratin 10 in superficial layer; while in OSF, skeletal muscle was found to be replaced with dense collagen I. These disease specific alterations were assumed to be the underlying phenomenon associated with intensity and textural variations in OCT images, using which specific quantitative imaging biomarkers were proposed.


 Please wait while we load your content...
Please wait while we load your content...