Thermally resistant unsaturated polyester resin with low dielectric loss based on special benzyl alcohol terminated hyperbranched polysiloxane for producing high efficiency motors using vacuum pressure impregnation technique†
Cheng Zhou,
Guozheng Liang* and
Aijuan Gu*
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Department of Materials Science and Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, China. E-mail: ajgu@suda.edu.cn; lgzheng@suda.edu.cn; Fax: +86 512 65880089; Tel: +86 512 65880967
First published on 11th January 2016
Abstract
Motors with higher efficiency and smaller size are the premise for producing higher performance electrical products and achieving an energy-saving world, so the motors should be fabricated with Vacuum Pressure Impregnation (VPI) technique and the matrices for the motors should be high performance resins that have higher thermal resistance and lower dielectric loss. However, available resins for common motors do not simultaneously have VPI processing characteristics, high thermal resistance and low dielectric loss. Herein, based on unsaturated polyester (UP), the common VPI resin for which the thermally resistant level is as low as F level (155 °C), a unique high performance resin is developed by co-polymerizing UP resin with a novel benzyl alcohol terminated hyperbranched polysiloxane (Vi-HPSi). The structure and integrated performances of Vi-HPSi/UP resins were intensively studied. Results show that Vi-HPSi/UP resin not only meets the strict processing requirements of VPI technique, but also has better curing features, thus endowing Vi-HPSi/UP resins with higher crosslinking density and reduced free volume than the UP resin. In addition, compared with the UP resin, Vi-HPSi/UP resins have much better toughness and bond strength, greatly improved thermal stability, and reduced dielectric loss. Typically, for the Vi-HPSi/UP resin with 20 wt% Vi-HPSi (20Vi-HPSi/UP), its initial degradation temperature is as high as 331 °C, about 80 °C higher than that of the UP resin, and this is also the highest value among modified UP resins reported; while the dielectric loss of 20Vi-HPSi/UP is about 0.62 times of that of the UP resin. These attractive performances demonstrate that the Vi-HPSi/UP resin has great potential for fabricating new generation motors with higher efficiency for cutting-edge applications.
1. Introduction
Nearly 80% of electrical products in the world are driven by motors, and about 80% of the energy in the world is consumed by motors,1 so motors with high efficiency and small size are the premise for producing higher performance electrical products and achieving an energy-saving world.Insulating materials are the heart of motors, high efficiency motors should be prepared by using high performance resins. In addition, the operation reliability and service life of motors are closely related to the insulation impregnating process. Vacuum Pressure Impregnation (VPI) has been regarded as the most advanced insulation impregnating process at present,2 which can prepare insulation structures without an air gap, and thus guarantee desirable electrical properties and insulation life for motors with the biggest possibility when the insulating materials are fixed.3,4 However, different from other conventional techniques, VPI technique asks for special processing requirements for resins, including very low viscosity (<500 mPa s at 25 °C) and excellent storage stability (>6 months).5 Note that the resins suitable for the VPI process generally do not have high performances.6 Therefore, the resin that has both outstanding service performances and VPI processing feature is the key to produce new generation highly efficient motors.
Unsaturated polyester (UP) is the main type resin for producing low voltage motors through VPI technique, which has low cost, fast curing speed and insulation performance.7–9 However, the heat-resistance class of available UP resins is only F class (155 °C),10,11 does not meet the increasing development on new motors with high efficiency and small size in recent years.
To prepare heat-resistant UP resin, many methods were used. Although the addition of inorganic fillers could improve the thermal resistance, the hybridized resins generally do not show good storage stability due to the aggregation and settlement of fillers.12 This phenomenon is avoided in organic method. The modified UP resin by imine alcohol (coded as PEI system) has been proved to be the most effective route to improve the heat-resistance of UP resin, but this generally declines the curing speed and increases dielectric loss.13 Therefore, it is still a great challenge to develop high performance UP resins for fabricating high efficient motors, that is, the resins not only maintain good processing feature for VPI technique, but also have significantly improved thermal resistance and reduced dielectric loss.
Hyperbranched polymers have attracted increasing attentions in recent years owing to their unique physical and chemical properties derived from their special structures.14 Some researches show that the addition of suitable contents of hyperbranched polysiloxane can enhance the heat-resistance of epoxy,15 cyanate ester resins,16,17 bismaleimide18 and polyamine,19 etc. However, the reported hyperbranched polysiloxanes are not suitable for VPI technique because excess alkoxy groups at the end of molecular chains will release volatile alcohol during curing, producing a large number of micro air defects in crosslinked networks20 and thus deteriorated the integrated performances.
This paper reports a new benzyl alcohol terminated hyperbranched polysiloxane with reactive double bonds (Vi-HPSi), of which polysiloxane chains and benzene rings provide outstanding thermal resistance,21 while reactive double bonds endow good curing reaction with UP resin. Based on the synthesis of Vi-HPSi, a series of Vi-HPSi/UP resins were prepared, which are proved to be suitable for fabricating high performance motors through VPI technique. The origin behind was revealed based on intensively investigating the structure–performance relationship.
2. Experimental
2.1 Materials
UP was supplied by Suzhou Jufeng Electrical Insulation System Co., Ltd, China. Styrene was bought from Changzhou Tianyun Chemical Co., Ltd, China. 1,4-Butanediol dimethacrylate was made in Shanghai Hechuang Chemical Co., Ltd, China. 3-(Trimethoxysilyl) propyl methacrylate (KH570) was supplied by Nanjing Shuguang Silane Chemical Co., Ltd, China. Stannous chloride was provided by Wenzhou Haisheng Chemical Co., Ltd, China. Dicumyl peroxide was bought from Shanghai Yongsheng Chemical Co., Ltd, China. p-tert-Butylcatechol and hydroquinones were purchased from Nanjing Shengfeng Chemical Co., Ltd, China. Distilled water was produced in our lab. All these materials were used as received.2.2 Synthesis of Vi-HPSi
KH570 (1673 g) and distilled water (170 g) were put into a three-necked flask equipped with a thermometer and condenser to form a solution. HCl was slowly dropped into the flask with stirring to adjust the pH value of the solution within 1 and 2. After maintained at room temperature for 20 min, the solution was heated to 50 °C and maintained at that temperature with stirring for 1 h. Then the flask was heated to 70 °C and stayed for 4 h. After that, the resultant product was treated under vacuum condition to remove redundant methanol, code as HPSi.Benzyl alcohol (663 g), stannous chloride (0.87 g) and hydroquinone (1.46 g) were added into above flask, and then the flask was heated and maintained at 150 °C for 5 h, followed by treating under vacuum condition at 200 °C to remove redundant methanol and benzyl alcohol. After that, a transparent viscous liquid was obtained, which was Vi-HPSi.
2.3 Preparation of UP resin
UP resin (500 g) was heated to 80 °C, into which 132 g styrene and 198 g 1,4-butanediol dimethacrylate were added with vigorous stirring; the mixture was stayed at 80 °C for 0.5 h, and then cooled down to 50 °C. After that 8.3 g dicumyl peroxide and 0.5 g p-tert-butylcatechol were added. The blend was maintained at 50 °C for 1 h with stirring, and then filtered with 300 mesh strainer. The resultant resin was UP resin.2.4 Preparation of Vi-HPSi/UP resins
Appropriate amounts of Vi-HPSi and UP were thoroughly blended at room temperature for 1 h to get new UP resin, which was coded as xVi-HPSi/UP, where x was the loading of Vi-HPSi, taking values of 5, 10, 15, and 20 wt%.2.5 Preparation of cured Vi-HPSi/UP, UP and Vi-HPSi resins
A steel mould (about φ 10 mm) was coated with a thin layer of silicone grease, and then heated and maintained at 200–230 °C for 4–5 h. After cooling down to room temperature, 10.0 ± 0.5 g resin (Vi-HPSi/UP or UP resin) was put into the steel mould. The mold was then put into an oven for curing with a procedure of 150 °C/6 h.Dicumyl peroxide and Vi-HPSi (the mass ratio of Vi-HPSi to dicumyl peroxide was 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were thoroughly blended at room temperature for 20 min, and then above curing procedure for curing Vi-HPSi/UP was used to cure Vi-HPSi.
3) were thoroughly blended at room temperature for 20 min, and then above curing procedure for curing Vi-HPSi/UP was used to cure Vi-HPSi.
2.6 Measurements
Fourier Transform Infrared (FTIR) spectra were recorded on a Prostar LC240 Infrared Spectrometer (USA).Si-Nuclear Magnetic Resonance (29Si-NMR) spectra were obtained using a Bruker 500 MHz instrument (Germany) with 128 scans at 99.36 MHz. CDCl3 was used as the solvent.
Gel Permeation Chromatography (GPC) measurements were carried out at 35 °C on an Agilent 1100 system (USA) with tetrahydrofuran as the eluent (1.0 mL min−1), and polymethyl methacrylate as the standard.
Viscosities were tested with a Brookfield viscometer CAP 2000+ (USA) at 25 °C.
Differential Scanning Calorimeter (DSC) measurements were performed with a DSC 200 F3 (NETZSCH, Germany) at a heating rate of 5 °C min−1 under a nitrogen atmosphere.
Gel time was measured using a glass test tube in a temperature-controlled oil bath pan.
Insulation resistance was tested on PC68 Digital Display High Resistance Tester (Shanghai Precision Scientific Instrument Co., Ltd, China).
Positron Annihilation Lifetime Spectra (PALS) were measured at 20 ± 0.5 °C under air atmosphere on an EG&G ORTEC fast–fast lifetime spectrometer (ORTEC Co., Tennessee, USA) with a FWHM = 190 psec for a 60Co prompt peak of 1.18 and 1.33 MeV γ rays. A positron source (22Na) of 6 × 105 Bq was deposited between two Kapton films (3 mm in thickness), which was sandwiched between two identical resin samples. Every spectrum contained about 106 counts. The resulting spectra were consistently modelled with a three-component fit with the computer program PATFIT-88.
Bond strengths were measured using RGM-4050 Mechanical Universal Testing Machine (Shenzhen Reger Instrument Co., Ltd, China) with solenoid coil hanging paint two times in opposite directions. The resin was cured at 150 °C for 4 h after first hanging paint, which was then hanged paint in opposite direction and cured at 150 °C for 6 h.
Thermogravimetric (TG) analyses were performed on a TG 209 F3 (NETZSCH, Germany) from 25 to 700 °C under a nitrogen atmosphere with a flow rate of 100 mL min−1 and a heating rate of 10 °C min−1.
Dielectric losses were tested using a QS30-2 Dielectric Loss Tester (Haikang Electronic Co., Ltd, China). According to the International Standard on Guide for the permittivity and dielectric dissipation factor of electrical insulation materials at power, audio and radio frequencies including meter wavelengths (IEC 60250-1969), the dielectric losses of insulation materials used in motor field are usually shown in percentage (%).
3. Results and discussion
3.1 Design and characterization of Vi-HPSi
Resins for fabricating motors should have compact structure, so the release of small molecules should be avoided during curing.22 To achieve this target, new hyperbranched polysiloxane with a large number of reactive double bonds and rigid benzene rings at the end of molecular chains was designed as shown in Fig. 1a. | ||
| Fig. 1 Synthesis of Vi-HPSi (a); FTIR spectra of KH570, HPSi, benzyl alcohol and Vi-HPSi (b); the 29Si-NMR spectrum of Vi-HPSi (c). | ||
Fig. 1b shows FTIR spectra of KH570, HPSi, benzyl alcohol and Vi-HPSi. Compared with the spectrum of KH570, that of HPSi shows typical vibrations assigning to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (1640 cm−1),
CH2 (1640 cm−1), ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Si
Si–O–Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) (1012–1125 cm−1) and
(1012–1125 cm−1) and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH (3460 cm−1), suggesting that methoxyl groups have been successfully changed into Si–O– through hydrolysis. These peaks are also found in the spectrum of Vi-HPSi; besides, the peaks representing benzene ring (1500 and 700 cm−1) also appear, demonstrating that HPSi has been terminated by benzyl alcohol.
Si–OH (3460 cm−1), suggesting that methoxyl groups have been successfully changed into Si–O– through hydrolysis. These peaks are also found in the spectrum of Vi-HPSi; besides, the peaks representing benzene ring (1500 and 700 cm−1) also appear, demonstrating that HPSi has been terminated by benzyl alcohol.
DB and ANB are two important parameters to describe the branching structures of hyperbranched polymers and are often calculated based on the NMR spectrum.23 A hyperbranched polymer prepared from AB3 monomer contains three different units such as dendritic unit (D), linear unit (L), and terminal unit (T). If D, L, and T represent the integral of chemical shifts for dendritic, linear, and terminal units, respectively, in the 29Si-NMR spectrum of the hyperbranched polymer, DB and ANB can be severally calculated from eqn (1) and (2).24 The 29Si-NMR spectrum of Vi-HPSi is shown in Fig. 1c. The integral values of D and L unit were calculated to be 1446.03 and 1984.8, and then DB and ANB of Vi-HPSi are 0.59 and 0.42 respectively.
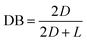 | (1) |
 | (2) |
The weight-average molecular weight (MW) and polydispersity (PDI) of HPSi and Vi-HPSi were measured by GPC technique. The MW and PDI values of HPSi are 3608 g mol−1 and 1.244, respectively; while those of Vi-HPSi are severally 6280 g mol−1 and 1.319.
3.2 Processing characteristics of Vi-HPSi/UP resin
To meet the processing feature of VPI technique, the resin should have suitably low viscosity (<500 mPa s at 25 °C)25 and long storage stability.26 The dependence of viscosity on temperature for Vi-HPSi/UP and UP resins is depicted in Fig. 2. It can be seen that Vi-HPSi/UP resins have slightly higher viscosity than UP resin at the same temperature, but the viscosities of 5Vi-HPSi/UP and 20Vi-HPSi/UP are only 320 and 356 mPa s at 25 °C, respectively, meeting the requirement of VPI process.In actual industrial process, a large quantity of VPI resins (more than 3 tons) are usually stored in a VPI tank, so the VPI resin should maintain low viscosity and uniformity to guarantee the good quality of impregnation.27 This is the meaning of good storage stability. The multiple of viscosity growth of resins is often used to evaluate the storage stability for VPI process, which is usually requested to be less than 0.5.28 Fig. 3 shows the multiples of viscosity growth of Vi-HPSi/UP resins after stored at different temperatures for 96 h. It can be seen that the multiples of viscosity growth for all Vi-HPSi/UP prepared are good for VPI technique. The multiple of viscosity growth for Vi-HPSi/UP system increases as either the temperature or the loading of Vi-HPSi increases, this is expected because the curing reaction takes place faster at higher temperature, and Vi-HPSi has slightly bigger viscosity than UP resin.
3.3 Curing behaviour and mechanism of Vi-HPSi/UP resins
The performances of a thermosetting resin are well known to be determined by the chemical and aggregation structures of its crosslinked network,29 both structures are greatly dependent on the curing behavior and mechanism.30Fig. 4 gives DSC curves of UP and Vi-HPSi/UP. UP shows a single and big exothermic peak from 105 to 216 °C. Similar peak also appears in each curve of Vi-HPSi/UP system, but shifts toward low temperature. Specifically, the maximum curing temperature of Vi-HPSi/UP resins significantly shifts to lower temperature with a gap of about 21–29 °C compared with that of UP resin, demonstrating that the whole curing process of UP can be significantly accelerated by a small amount of Vi-HPSi. The reason is attributed to the curing mechanism.
The curing mechanism of UP resin is the copolymerization of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–.31 This is also included in that of Vi-HPSi/UP system, however, Vi-HPSi owns spherical structure, and has a large number of terminal double bonds (–HC
CH–.31 This is also included in that of Vi-HPSi/UP system, however, Vi-HPSi owns spherical structure, and has a large number of terminal double bonds (–HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). Note that compared with –CH
CH2). Note that compared with –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, –HC
CH–, –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 has higher reactivity,32 and will copolymerize with double bonds of UP (Fig. 5), leading to faster curing reaction for Vi-HPSi/UP system.
CH2 has higher reactivity,32 and will copolymerize with double bonds of UP (Fig. 5), leading to faster curing reaction for Vi-HPSi/UP system.
Gel time is the time required for the resin to stop legging and becomes elastic,33 so it is a more direct parameter for evaluating the processing feature for VPI technique. As shown in Fig. 6, all Vi-HPSi/UP have obviously shorter gel time than UP at the same temperature, especially when the temperature is below 120 °C, implying that Vi-HPSi/UP system exhibits higher reaction activity and faster curing speed, this is consistent with above DSC results.
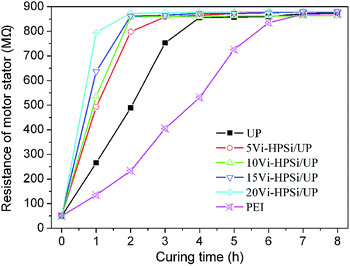
The resistant stability that means complete curing of a resin will provide further characterization on the curing speed of resin in practical application. Fig. 7 gives the resistance of motor stators that were impregnated with different resins during curing at 150 °C. It can be seen that the resistance of the motor stator impregnated with UP is stable after 4 h, while that with Vi-HPSi/UP increases rapidly and becomes stable after stayed for 2 to 3 h, suggesting that Vi-HPSi/UP has fast curing speed, this is beneficial to shorten working period and save energy. Previous research showed that the resistance of the motor stator impregnated with PEI system (UP modified with 15 wt% imine alcohol) becomes stable about stayed for 7 h, showing decreased curing speed.34 Therefore, the Vi-HPSi/UP resins developed herein have super advantages in obtaining good processing feature.
3.4 Crosslinked structures of Vi-HPSi/UP resins
Crosslinking density and free volume are important property to characterize the aggregation state structure of a thermosetting resin.35,36 The crosslinking densities of original and modified UP resins are depicted in Fig. 8a. All Vi-HPSi/UP resins have higher crosslinking density than UP resin, it is reasonable because the large number of terminal double bonds of Vi-HPSi not only provide more curing points, but also promote the curing reaction of UP as proved above. These facts also explain the phenomenon that crosslinking density increases as the Vi-HPSi content increases, and reaches the maximum at 15 wt% of Vi-HPSi. When the loading of Vi-HPSi is too high, the cavity structure of Vi-HPSi plays a bigger role and offsets the parameter for increasing crosslinking density.Fig. 8b gives the average size of free volume cavities (Vh) and number of free volume (fapp) of UP and Vi-HPSi/UP resins calculated according to the o-Ps lifetime (τ3) and o-Ps intensity (I3) directly obtained from PALS tests (Fig S1 in ESI†). With the addition of Vi-HPSi into UP resin, both Vh and fapp reduce at first and then gradually increase as the content of Vi-HPSi increases. This result further confirms that Vi-HPSi/UP resins have different microstructure from UP resin. Two opposite factors, crosslinking density and cavity structure, are responsible for the dependence of free volume on the loading of Vi-HPSi. Specifically, higher crosslinking density will reduce free volume cavities, while the cavity structure of Vi-HPSi increases free volume cavities.
3.5 Integrated performances of EPEI/HSi–TiO2 hybrids
All Vi-HPSi/UP resins have about 1.1–1.2 times higher bond strengths than UP resin. The improved bond strength is derived from two reasons. Firstly, reactive double bonds and Si–OH groups of Vi-HPSi improve the interfacial adhesion between the resin and copper wires. Secondly, the higher toughness of Vi-HPSi/UP resins is beneficial to prevent the crack spread. As the two influences are related to the loading of Vi-HPSi, so the bond strength is also related to the loading of Vi-HPSi in the modified resin.
 | ||
| Fig. 10 DSC curves of cured UP and Vi-HPSi/UP resins (a); TG and DTG curves of cured Vi-HPSi, UP and Vi-HPSi/UP resins (b and c). | ||
Overlay TG curves of cured UP, Vi-HPSi and Vi-HPSi/UP resins are shown in Fig. 10b. Vi-HPSi/UP resins have obviously higher initial decomposition temperature (Tdi) values than UP resin, for example, with 10 or 20 wt% addition of Vi-HPSi, the Tdi value is 306 or 331 °C, about 55 or 80 °C higher than that of UP resin, so Vi-HPSi/UP resins have much better thermal stability than neat UP resin because Tdi is regarded as the index for evaluating the thermal stability of materials.38,39 It is worth mentioning that among reports on developing thermally resistance of UP resin, Vi-HPSi/UP resin prepared herein has the highest Tdi after comparing the Tdi values of different UP resin systems.40,41 The phenomenon is mainly attributed to the excellent thermal stability of cured Vi-HPSi, of which Tdi is 358 °C, about 107 °C higher than that of UP resin. On the other hand, Fig. 10b also shows that all Vi-HPSi/UP resins have much higher char yield (Yc) at 700 °C than UP resin, and a larger loading of Vi-HPSi leads to greater Yc, reflecting that Vi-HPSi can promote the char formation due to high Yc of Si–O–Si chains and benzene rings, and thus providing greatly improved ability of protecting the resin from further degradation.
 | ||
| Fig. 11 Dielectric losses at different frequencies (a) and temperatures (b) for cured UP, Vi-HPSi/UP and PEI resins. | ||
Generally, dielectric properties of polymers depend on the orientation and relaxation of dipoles in the applied electric field, the process of dipole polarization is accompanied with the movement of polymer chain segments,43 so the attractively reduced loss of Vi-HPSi/UP resins is attributed to the greatly different structure between UP and Vi-HPSi/UP resins. Briefly, Vi-HPSi has low dielectric loss due to the large amount of polysiloxane18 and benzene rings. In addition, Vi-HPSi/UP resins have higher crosslinking density, tending to provide a great restricting influence on the orientation and relaxation of dipoles in the applied electric field, and thus decreasing the dielectric loss.
4. Conclusions
Through hydrolysis and blocking reaction, a new benzyl alcohol terminated hyperbranched polysiloxane (Vi-HPSi) was designed and synthesized. Vi-HPSi/UP system has low viscosity and good storage stability, meeting harsh requirements of VPI technique. Vi-HPSi can effectively promote the curing reaction and change the microstructure of UP. Compared with UP resin, cured Vi-HPSi/UP resin simultaneously has outstanding thermal stability, mechanical strength and remarkably decreased dielectric loss, showing great potential in fabricating high efficient motors. These attractive performances of Vi-HPSi/UP resins demonstrate that the method for preparing Vi-HPSi and related modification is an effective and promising approach for developing high performance VPI resins.Acknowledgements
We thank National Natural Science Foundation of China (21274104), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Science and Technology Support Plan of Jiangsu Province, China (BE2013848) and Nano Special Technology of Suzhou City, China (ZXG2013007) for finically supporting this project.References
- I. Kortas, A. Sakly and M. F. Mimouni, Energy, 2015, 89, 55 CrossRef.
- B. J. Zhang, A. J. Gu, G. Z. Liang, J. T. Hu, D. X. Zhuo and L. Yuan, Polym. Adv. Technol., 2011, 22, 2415 CrossRef CAS.
- D. Ghosh, S. Bhandari, T. K. Chaki and D. Khastgir, RSC Adv., 2015, 5, 57608 RSC.
- X. Yu, W. Wu, W. Pan, S. Han, L. Wang and J. Wei, IEEE Trans. Appl. Supercond., 2012, 22, 7700504 CrossRef.
- T. Weiers, Y. Corrodi, R. Brutsch and R. Vogelsang, IEEE 8th International Conference on Properties and Applications of Dielectric Materials, 2006, Bali Search PubMed.
- C. X. Hu, Y. G. Man and X. H. Zhang, Appl. Mech. Mater., 2012, 1682, 121–126 CrossRef.
- H. Kargarzadeh, R. M. Sheltami, I. Abdullah and A. Dufresne, Polymer, 2015, 56, 346 CrossRef CAS.
- K. Kanimozhi, P. Prabunathan, V. Selvaraj and M. Alagar, RSC Adv., 2014, 4, 18157 RSC.
- L. Q. Ma and L. Y. Yu, Insul. Mater., 2004, 6, 36 Search PubMed.
- B. A. Zaitsev, L. G. Kleptsova, I. D. Shvabskaya and O. V. Sorochinskaya, Russ. J. Appl. Chem., 2012, 85, 969 CrossRef CAS.
- B. K. Kandola, L. Krishnan, D. Deli, P. Luangtriratana and J. R. Ebdon, RSC Adv., 2015, 5, 33772 RSC.
- F. Ansari, M. Skrifvars and L. Berglund, Compos. Sci. Technol., 2015, 117, 298 CrossRef CAS.
- W. Y. Chiang and W. C. Chiang, J. Appl. Polym. Sci., 1988, 35, 1433 CrossRef CAS.
- H. J. Yang, J. B. Xu, S. Pispas and G. Z. Zhang, RSC Adv., 2013, 3, 6853 RSC.
- D. Ratna and G. P. Simon, Polym. Eng. Sci., 2001, 41, 1815 CAS.
- Z. Y. Zhang, L. Yuan, G. Z. Liang, A. J. Gu, Z. X. Qiang, C. W. Yang and X. X. Chen, J. Mater. Chem. A, 2014, 2, 4975 CAS.
- C. Zhou, A. J. Gu, G. Z. Liang and L. Yuan, Polym. Adv. Technol., 2011, 22, 710 CrossRef CAS.
- D. X. Zhuo, A. J. Gu, G. Z. Liang, J. T. Hu, L. Yuan and X. X. Chen, J. Mater. Chem., 2011, 21, 6584 RSC.
- Z. X. Qiang, G. Z. Liang, A. J. Gu and L. Yuan, Mater. Lett., 2014, 115, 159 CrossRef CAS.
- D. Evans, J. Knaster and H. Rajainmaki, IEEE Trans. Appl. Supercond., 2012, 22, 4202805 CrossRef.
- Z. J. Jiang, L. Yuan, G. Z. Liang and A. J. Gu, Polym. Degrad. Stab., 2015, 121, 30 CrossRef CAS.
- Y. Luo, G. N. Wu, J. W. Liu, G. Y. Zhu, P. Wang, J. Peng and K. J. Cao, IEEE Trans. Dielectr. Electr. Insul., 2014, 21, 2237 CrossRef CAS.
- R. K. Pandey, M. D. Hossain, T. Sato, U. Rana, S. Moriyama and M. Higuchi, RSC Adv., 2015, 5, 49224 RSC.
- L. J. Markoski, J. L. Thompson and J. S. Moore, Macromolecules, 2002, 35, 1599 CrossRef CAS.
- C. P. Zeng, Y. Xia and W. Wang, Electrical Manufacturing and Coil Winding Expo, Milwakee, 2013 Search PubMed.
- S. Jahromi, Polymer, 1999, 40, 5103 CrossRef CAS.
- N. Frost, M. Winkeler and S. Tuckwell, Electrical Insulation Conference (EIC), Seattle, 2015 Search PubMed.
- W. Golbig, Elektrie, 1997, 51, 446 Search PubMed.
- N. Régnier, J. Berriot, E. Lafontaine and B. Mortaigne, Polym. Degrad. Stab., 2001, 73, 485 CrossRef.
- Y. Nakamura and S. Yamago, Macromolecules, 2015, 48, 6450 CrossRef CAS.
- L. Keller, C. Decker, K. Zahouily, S. Benfarhi, J. M. le Meins and J. Miehe-Brendle, Polymer, 2004, 45, 7437 CrossRef CAS.
- S. Ghosh, S. K. Goswami and L. J. Mathias, J. Mater. Chem., 2013, 1, 6073 RSC.
- M. B. Salehi, M. V. Sefti, A. M. Moghadam and A. D. Koohi, J. Macromol. Sci., Part B: Phys., 2012, 51, 438 CrossRef CAS.
- S. Z. Guo, S. Z. Yuan, Y. Xia and Z. N. Hu, Insul. Mater., 2012, 45, 9 CAS.
- J. Jancar, J. Mater. Sci., 2008, 43, 6747 CrossRef CAS.
- Y. V. Kulvelis, S. S. Ivanchev, V. T. Lebedev, O. N. Primachenko, V. S. Likhomanov and G. Török, RSC Adv., 2015, 5, 73820 RSC.
- Y. N. Tang, L. Yuan, G. Z. Liang and A. J. Gu, RSC Adv., 2014, 4, 16136 RSC.
- D. L. F. José L., R. B. Marta, M. S. César and O. E. Susana, Polym. Degrad. Stab., 2011, 96, 943 CrossRef.
- K. Bauri, S. G. Roy, S. Arora, R. K. Dey, A. Goswami, G. Madras and P. de, J. Therm. Anal. Calorim., 2013, 111, 753 CrossRef CAS.
- G. R. Peng, Q. S. Li, Y. L. Yang and H. F. Wang, J. Appl. Polym. Sci., 2009, 114, 2128 CrossRef CAS.
- S. Z. Guo, S. Z. Yuan, Y. Xia and Z. N. Hu, Insul. Mater., 2012, 45, 9 CAS.
- M. Li, X. Y. Huang, C. Wu, H. P. Xu, P. K. Jiang and T. Tanaka, J. Mater. Chem., 2012, 22, 23477 RSC.
- B. H. Wang, Y. C. Jiao, A. J. Gu, G. Z. Liang and L. Yuan, Compos. Sci. Technol., 2014, 91, 8 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Results from positron annihilation lifetime spectra for UP and Vi-HPSi/UP resins. See DOI: 10.1039/c5ra23768h |
| This journal is © The Royal Society of Chemistry 2016 |