Tetracycline hydrochloride loaded regenerated cellulose composite membranes with controlled release and efficient antibacterial performance†
Wei Shao‡
*a,
Shuxia Wang‡a,
Xiufeng Liub,
Hui Liua,
Jimin Wua,
Rui Zhang*a,
Huihua Minc and
Min Huanga
aCollege of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, P. R. China. E-mail: w.shao@njfu.edu.cn; zhangrui@njfu.edu.cn; Fax: +86-25-85418873; Tel: +86-25-85427024
bJiangsu Key Laboratory of TCM Evaluation and Translational Research, China Pharmaceutical University, Nanjing 210009, P. R. China
cAdvanced Analysis and Testing Center, Nanjing Forestry University, Nanjing 210037, P. R. China
First published on 14th December 2015
Abstract
Fabrication of cellulose based composites with controlled release and efficient antibacterial performances is of general interest in biomedical areas. In this study, antibiotic drug, tetracycline hydrochloride (TCH), loaded regenerated cellulose (RC) composite membranes were prepared and their drug release, antibacterial activity and biocompatibility were evaluated. The structure and morphology of the formed RC-TCH composite membranes were characterized using scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) spectroscopy. The TCH release results showed that the RC membrane is capable of controlled release. In vitro antibacterial assay demonstrated that the developed RC-TCH composites displayed excellent antibacterial activity, solely associated with the loaded TCH drug. More importantly, the RC-TCH composites displayed good biocompatibility, thus confirming its utility as potential in wound dressings and other medical applications.
Introduction
Tetracycline hydrochloride (TCH) is a broad spectrum antibiotic with a common chemical structure and pharmaceutical actions, frequently used against gram-positive and gram-negative microorganisms.1,2 The structure of TCH is shown in Fig. 1. TCH can be used as skin and bone ointments to treat bacterial infections. Controlled release of TCH may benefit faster regeneration of osteoblasts, fibroblasts and other types of cells.3 TCH loaded poly(vinyl alcohol)/chitosan/ZrO2 hybrid nanofibers were fabricated by an electrospinning technique and showed well controlled release and great antibacterial activity.4 TCH loaded polycaprolactone nanofibers were prepared for sustained controlled drug delivery.3 The effects of TCH loaded silk fibroin membranes on the proliferation and the osteogenic differentiation of human mesenchymal stem cells were investigated and the results showed they are suitable for stem cell tissue engineering.5 TCH was loaded into poly(lactic acid), poly(caprolactone) and their blends via an electrospinning process to produce wound dressings, and the prepared nanofibrous mats presented a sustained and suitable drug-release rate, adequate water uptake and permeability, and antibacterial activities.6Cellulose, the most abundant renewable polymer on earth, has attracted much attention due to its unique properties, including biocompatibility, biodegradability, high mechanical strength, thermal and chemical stability.7 Cellulose is a linear polysaccharide composed of β-1-4-linked D-glucopyranose repeating units, and has been extensively used in various applications, such as medicine and electronics industries, energy areas as well as cosmetic and food industries.8,9 To explore new porous biomaterials in recent years, cellulose based materials are becoming of research interest for medical applications, especially for potential scaffold for tissue engineering and wound dressing areas.10–12 However, cellulose has a lack of inherent antibacterial properties, resulting in failure to provide a barrier against infection, which limits the possibilities of application in medical areas. One of the current therapies for treating bacterial infection relies on the systematic administration of antibiotics. However, it is often associated with toxicity due to the high concentration of antibiotics required.13 Therefore, controllable antibiotic delivery systems have been developed that it can reduce drug doses and eventually reduce the complications and side-effects. Most importantly, the drug could keep chemical stable in the presence of biological material and show controlled release behavior.14 Therefore, regenerated cellulose (RC) was fabricated with a fine fibrous network structure to load therapeutic drugs, which represent excellent pharmaceutical candidates.15 Moreover, the release of the therapeutic agent can increase the efficiency by being loaded into the cellulose matrices, including antibacterial agents,16–18 anti-cancer drugs,19–21 anti-inflammatory drugs,22 antibiotics,23,24 proteins25 and DNA.26,27
In this study, TCH loaded RC composite membranes were fabricated and their drug release behavior, antibacterial activity and cytotoxicity were evaluated. The formed RC-TCH composite membranes were characterized using scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) spectroscopy. The drug release behavior and antibacterial activities of the drug-loaded composite membranes were also investigated, respectively. The biocompatibility was investigated through dimethyl thiazolyl diphenyl (MTT) viability assay.
Experimental
Materials
Tetracycline hydrochloride (TCH, >98%) was purchased from Shanghai EKEAR Bio@Tech Co. Ltd. Microcrystal cellulose (MC, degree of polymerization up to 350) was purchased from Sinopharm Chemical Reagent Co., Ltd. The other chemicals used in the tests were purchased from Sinopharm Chemical Reagent Co., Ltd. All reagents were of analytical grade and used as received without further purification.Production of RC-TCH composite membranes
RC membranes were manufactured by a solution casting method using a tape template. A tape with 75 μm thickness was pasted on a sheet of glass plate and a rectangle template (15 × 10 cm) was made from the area within the tape. A transparent cellulose solution was prepared by dissolving 0.15 g MC powder (3 wt% of solution) into 5 g ZnCl2/H2O (3.5/1.5 by weight ratio) and heating at 90 °C for 2 h. The obtained cellulose solution was casted into the template on glass plate, and the whole glass plate was soaked in a de-ionized water bath for 30 min, followed by rinsing with de-ionized water. Then, the RC membranes underwent dialysis for one week to remove ZnCl2 and freeze-dried at −40 °C for 24 h.The RC-TCH composite membranes were prepared by immersing RC into a TCH solution with concentrations of 0.01, 0.05, 0.1, 0.3 and 0.5 g L−1 for 24 h at a gentle stirring of 20 rpm in the dark. Then, they were freeze-dried at −40 °C for 24 h. The final membranes were named as RC0.01, RC0.05, RC0.1 RC0.3 and RC0.5. The detailed procedure is listed in Scheme 1.
Characterization
A JSM-7600F Scanning Electron Microscope (SEM) operating at an accelerating voltage of 10–15 kV was used to investigate the surface morphologies of RC and RC-TCH composite membranes. The samples were coated with a thin layer of gold under high vacuum conditions (20 mA, 100 s). The elemental composition of the prepared RC membranes was analyzed with an energy dispersive X-ray spectroscope (EDS) coupled to the SEM.FTIR spectra were obtained on a Spectrum Two spectrometer (Perkin Elmer, USA) with the wavenumber range of 4000–400 cm−1 at a resolution of 4 cm−1.
TCH determination
TCH contents were calculated according to the original concentration of TCH (C0) and the concentration of unloaded TCH (C1) quantified using a SHIMADZU UV 2450 spectrophotometer at the monitoring wavelength of 356 nm. TCH weight percentages in the composite membranes were determined with the following equation:
 | (1) |
In vitro release assays
The tested composite membranes were cut into round pieces with a diameter of 10 mm. The release behaviors of TCH from the prepared RC-TCH composites were studied in PBS buffers with pH 7.4. The same amount of free TCH powders as TCH in RC0.5 was used as a control. The prepared samples were fully immersed in a beaker containing 50 mL 10 mM PBS at 37 °C and sealed using PARAFILM®M. At specific time points, an aliquot of 3.5 mL was collected from each solution and the absorbance was then measured at 356 nm by a SHIMADZU UV 2450 spectrophotometer. An equivalent volume of fresh PBS buffer was replaced into the system after each sampling to maintain constant medium volume. Thus, the concentrations of released TCH are obtained at different time can be calculated using the standard curve of the concentration vs. absorbance (data not shown). As a result, the cumulative concentrations can be calculated accordingly. The experiments were performed in triplicate.Antibacterial and antifungal activities
The antibacterial activities of RC-TCH composite membranes were investigated by a disk diffusion method against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 6538 and Candida albicans CMCC(F) 98001. RC-TCH composites and RC membrane (the control) were cut into round shapes with 10 mm diameter and sterilized by ultraviolet lamp for 60 min.Lawns of test bacteria (about 1 × 105 CFU per plate) were prepared on TSA. The sterilized samples were then carefully placed upon the lawns and RC was used as a control. The plates were placed in a 37 °C incubator for 24 h. Then, the inhibitory action of the tested samples on the growth of the bacteria was determined by measuring the diameter of the inhibition zone.
Cytotoxicity tests
The HEK293 cell lines were cultured in RPMI medium supplemented with 10% FBS, 100 μg mL−1 penicillin and 100 μg mL−1 streptomycin.The cytotoxicity was measured using the MTT assay method. 200 μL of HEK293 cells, at a density of 1 × 105, were placed in each well of a 48-well plate. Then, the cells were incubated over night at 37 °C in a humidified 5% CO2-containing atmosphere. Furthermore, the media was discarded. RC-TCH composite membranes with the same size (5 × 5 mm) were placed slightly on the top of cells and then fresh media was added. Wells containing only the cells were used as a control. The cells were treated for another 24 h. Then, the media containing sample was changed with fresh media and 20 μL of dimethyl thiazolyl diphenyl (MTT) was added and the incubation continued for 6 h. The medium was removed and 200 μL DMSO was added to each well to dissolve the formazan. The absorbance was measured with a test wavelength of 570 nm and a reference wavelength of 630 nm. Empty wells (DMSO alone) were used as blanks. The relative cell viability was measured by comparison with the control well containing only the cells. On the other hand, HEK293 cells were plated on the confocal culture dish. As cells reached to 30% confluence in all groups, then, the cells were treated with RC and RC0.5 for another 24 h. Cells were fixed and stained with FITC-phalloidin and DAPI. The morphologies were visualized by Confocal Microscopy (Leica DM2500, Germany).
Results and discussion
Characterization of RC-TCH composite membranes
The MC was dissolved completely in the ZnCl2 solution to become a transparent solution. After regeneration, EDS was applied to detect any Zn element left on the RC membranes and the result is listed in Fig. S1.† Zn was not shown in the EDS spectrum at all which indicating it is enough to remove ZnCl2 after one week dialysis in our study. Then, RC membranes were immersed into TCH solution to prepare RC-TCH composite membranes.The morphologies of the prepared pristine RC and RC0.5 membranes were analyzed using SEM (Fig. 2). Fig. 2A shows the morphology of RC that exhibited a nanoporous three-dimensional network structure with a random arrangement of ribbon-shaped microfibrils without any preferential orientation, resulting in a large surface area and high porosity of RC. In the case of the RC0.5 composites, TCH particles were displayed as white spots that can be easily found in the composites, resulting in a denser network structure, which is illustrated in the RC0.5 composite membrane (Fig. 2B). TCH weight percentages and contents were calculated and the results were listed in Table 1. It can be observed that the TCH loadings in the composite membranes increase with increasing original TCH concentrations. In addition, the thickness, mechanical property and porosity of the prepared RC composite membranes were listed in Table S1.† There is no significant difference in the thickness and mechanical property after TCH loaded in RC membranes.
| Samples | TCH (wt%) | TCH content (mg cm−2) |
|---|---|---|
| RC0.01 | 0.09 ± 0.01 | 0.028 ± 0.001 |
| RC0.05 | 0.17 ± 0.01 | 0.053 ± 0.001 |
| RC0.1 | 0.23 ± 0.03 | 0.075 ± 0.006 |
| RC0.3 | 0.88 ± 0.02 | 0.280 ± 0.027 |
| RC0.5 | 2.54 ± 0.04 | 0.810 ± 0.012 |
The dissolution and regeneration processes of cellulose are based on the disruption and formation of hydrogen, respectively. First, MC was dissolved by ZnCl2 aqueous solution to form homogeneous cellulose solution. When the formed cellulose solution was immersed into a de-ionized water (non-solvent), Zn2+ and Cl− bonded on the –OH groups of cellulose were removed out in water, and then the new –OH groups were regenerated, which result in the regeneration of cellulose. Due to the phase separation between solvent and non-solvent, many pores form in the cellulose gel, leading to a three-dimensional network structure.28
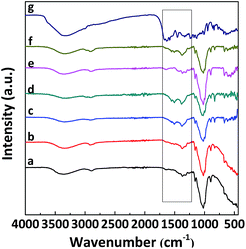
Fig. 3A displays the FTIR spectra of RC and RC-TCH composite membranes with different loadings of TCH. For RC (curve a), the FTIR spectrum was typical and the broad absorption band located from 3200 to 3500 cm−1 corresponds to the intramolecular hydrogen bond for 3O⋯H–O5 and the hydroxyl group.29,30 An absorption at 2900 cm−1 is due to CH2 groups.31 In the C–O stretching vibration region, the peaks at 1163 and 1061 cm−1 correspond to the C–O asymmetric bridge stretching and the C–O–C pyranose ring skeletal vibration, respectively.30 The FTIR spectrum of raw MC is shown in Fig. S2.† It was similar to the one of RC, which indicated that there was no significant difference of chemical structure between raw MC and RC and no chemical reaction had occurred during the dissolution and regeneration processes. The identifiable peaks of TCH (curve g) are C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of Amide I at 1669 cm−1, C
O vibration of Amide I at 1669 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of A-ring at 1634 cm−1, C
O vibration of A-ring at 1634 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of C-ring at 1581.0 cm−1, NH2 deformation of Amide II at 1535 cm−1 and C
O vibration of C-ring at 1581.0 cm−1, NH2 deformation of Amide II at 1535 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration of aromatic ring at 1456 cm−1.32 For RC-TCH composites (curves b–f), the peak intensities of TCH becomes more evident with TCH loading increasing in the spectra of RC-TCH composites, which verifies the existence of TCH in the composite membranes.
C vibration of aromatic ring at 1456 cm−1.32 For RC-TCH composites (curves b–f), the peak intensities of TCH becomes more evident with TCH loading increasing in the spectra of RC-TCH composites, which verifies the existence of TCH in the composite membranes.
Release behavior in vitro
To evaluate the release behavior of TCH from RC-TCH composite membranes, cumulative release profiles were monitored in the PBS buffers at pH 7.4 and the results are shown in Fig. 4. The calculated release rates of different loadings of TCH are shown in Fig. S3.† There is an initial burst release for all tested specimen and then they displayed a gradually decreased release of TCH. Eventually, RC-TCH composite membranes showed a steady release rate of TCH. The release rate of TCH increased with the TCH loading increasing in the RC membrane. As expected, free TCH released much faster than RC-TCH composite membranes, whereas TCH loaded into RC membrane can clearly slow the release activity. Thus, RC membrane was proved to be an excellent TCH carrier to control its release behavior. | ||
| Fig. 4 Cumulative release profiles of TCH in the PBS buffer at pH 7.4 (curves a–f are RC0.01, RC0.05, RC0.1, RC0.3, RC0.5 and free TCH (the same amount as RC0.5)). | ||
Antibacterial activity
TCH has a broad antibiotic spectrum that includes Gram-negative and Gram-positive bacteria. Three strains, including Gram-negative E. coli ATCC 25922, Gram-positive S. aureus ATCC 6538 and fungus C. albicans CMCC(F)98001, were selected as model bacteria and fungus for antibacterial tested because they are usually associated with the infections during wound healing procedure.33–35The antibacterial activities of the RC and RC-TCH composite membranes were investigated by a disc diffusion method. The prepared composites were placed on a lawn of tested bacteria in TSA, respectively. The antibacterial activity is measured by the clear zone of inhibition around the samples after 24 h incubation and the images are shown in Fig. 5. As expected, no inhibition zones was observed for RC as control (a), implying that RC do not have any antibacterial properties against the tested three strains.
 | ||
| Fig. 5 Optical images of inhibition zones of RC and RC-TCH composite membranes: (A) E. coli, (B) S. aureus and (C) C. albicans. (In all plates, (a–f) are RC, RC0.01, RC0.05, RC0.1, RC0.3 and RC0.5). | ||
The average diameters of inhibition zones of prepared RC and RC-TCH composites evaluated from the disc diffusion method are listed in Fig. 6. With increasing TCH loadings in the composite, the inhibition zones increase rapidly first and then trend to be stable. RC0.5 has the best antibacterial activities that its zone of inhibition diameter of E. coli and S. aureus are 28.5 and 30.4 mm, respectively, whereas RC0.5 has a much smaller diameter of inhibition zone of 17.4 mm against C. albicans. The present study clearly indicates that RC-TCH composite membranes show excellent antibacterial activities.
 | ||
| Fig. 6 Average diameters of inhibition zones of RC-TCH composite membranes, includes disk diameter of 10 mm: (A) E. coli, (B) S. aureus and (C) C. albicans. | ||
Cytotoxicity of RC-TCH composite membranes
Cellular growth and proliferation on surfaces are a symbol of cytocompatibility for materials that can be used to assess the potential of materials for application in tissue engineering.36 Cytotoxicity studies were performed to investigate the effect of TCH in the RC matrix on proliferation of HEK293 cell line. The effect of RC, without TCH, was evaluated in vitro to ensure that TCH did not have an independent toxicity effect. The cell viability of HEK293 cells was evaluated by MTT assay. The cell cytotoxicity imparted by RC-TCH composites being placed on the top of HEK293 cells was studied. HEK293 cells were placed in each well of a 48-well plate and incubate for 24 h. The MTT results were illustrated in Fig. 7 as relative viability of the cells by comparison with the control well containing only the cells. All the materials showed negligible toxicity. No reduced cell viability following their incubation with RC-TCH composites was shown. The results showed that TCH do not inhibit the proliferation of HEK293 cells, even at a high concentration because HEK293 cells do not seem to be affected from their incubation with RC-TCH composites.To determine whether RC-TCH composite membranes affect the morphology of HEK293 cells, the cells were stained and observed by fluorescence microscopy (Fig. 8). The morphologies of HEK293 cells treated with RC and RC0.5 membranes were similar to the blank one, which proves the prepared RC-TCH composite membranes have no effect on HEK293 cells morphology at all. These results show that RC-TCH composite membranes are promising candidates for wound dressing and tissue engineering applications.
Conclusions
In summary, controlled release and antibacterial activity of TCH loaded RC composite membranes were prepared and investigated. TCH loaded RC displayed a denser network structure compared to the three-dimensional network structure of RC. RC-TCH membranes could release the drug in a sustained manner, displaying a steady release after an initial burst release. RC-TCH composite membranes possess excellent biocompatibility and display effective antibacterial activity against E. coli, S. aureus and C. albicans. Therefore, the developed TCH loaded RC composite membranes have potential applications in wound dressing and tissue engineering.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (51401109), the High-level Talent Project of Nanjing Forestry University (GXL201301), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors would like to thank the Advanced Analysis & Testing Center of Nanjing Forestry University.Notes and references
- M. E. Parolo, M. J. Avena, G. Pettinari, I. Zajonkovsky, J. M. Valles and M. T. Baschini, Appl. Clay Sci., 2010, 49, 194–199 CrossRef CAS
.
- F. Quero, M. Nogi, K. Y. Lee, G. Vanden Poel, A. Bismarck, A. Mantalaris, H. Yano and S. J. Eichhorn, ACS Appl. Mater. Interfaces, 2011, 3, 490–499 CAS
.
- M. Rouabhia, J. Asselin, N. Tazi, Y. Messaddeq, D. Levinson and Z. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 1439–1446 CAS
.
- H. L. Wang, C. J. Chu, L. L. Hao, Y. She, Y. A. Li, L. F. Zhai and S. T. Jiang, J. Appl. Polym. Sci., 2015, 132, 42506 Search PubMed
.
- S. H. Jin, H. Y. Kweon, J. B. Park and C. H. Kim, J. Surg. Res., 2014, 192, E1–E9 CrossRef CAS PubMed
.
- P. Zahedi, Z. Karami, I. Rezaeian, S. H. Jafari, P. Mahdaviani, A. H. Abdolghaffari and M. Abdollahi, J. Appl. Polym. Sci., 2012, 124, 4174–4183 CrossRef CAS
.
- J. Chen, Y. Guan, K. Wang, X. Zhang, F. Xu and R. Sun, Carbohydr. Polym., 2015, 128, 147–153 CrossRef CAS PubMed
.
- A. M. d. A. Júnior, G. Braido, S. Saska, H. S. Barud, L. P. Franchi, R. M. N. Assunção, R. M. Scarel-Caminaga, T. S. O. Capote, Y. Messaddeq and S. J. L. Ribeiro, Carbohydr. Polym., 2016, 136, 892–898 CrossRef PubMed
.
- Y. Song, Y. Zhou, S. van Drunen Littel-van den Hurk and L. Chen, Biomater. Sci., 2014, 2, 1440 RSC
.
- M. I. Ahamed, S. Sankar, P. M. Kashif, S. K. Basha and T. P. Sastry, Int. J. Biol. Macromol., 2015, 72, 680–686 CrossRef CAS PubMed
.
- M. Soheilmoghaddam, M. U. Wahit, W. Tuck Whye, N. Ibrahim Akos, R. Heidar Pour and A. Ali Yussuf, Carbohydr. Polym., 2014, 106, 326–334 CrossRef CAS PubMed
.
- M. K. Joshi, H. R. Pant, A. P. Tiwari, B. Maharjan, N. Liao, H. J. kim, C. H. Park and C. S. Kim, Carbohydr. Polym., 2016, 136, 154–162 CrossRef CAS PubMed
.
- J. Song, J. C. Odekerken, D. W. Lowik, P. M. Lopez-Perez, T. J. Welting, F. Yang, J. A. Jansen and S. C. Leeuwenburgh, Macromol. Biosci., 2015, 15, 901–911 CrossRef CAS PubMed
.
- N. Monteiro, M. Martins, A. Martins, N. A. Fonseca, J. N. Moreira, R. L. Reis and N. M. Neves, Acta Biomater., 2015, 18, 196–205 CrossRef CAS PubMed
.
- A. Duro-Castano, J. Movellan and M. J. Vicent, Biomater. Sci., 2015, 3, 1321–1334 RSC
.
- S. Gustaite, J. Kazlauske, J. Bobokalonov, S. Perni, V. Dutschk, J. Liesiene and P. Prokopovich, Colloids Surf., A, 2015, 480, 336–342 CrossRef CAS
.
- W. Shao, H. Liu, X. Liu, S. Wang, J. Wu, R. Zhang, H. Min and M. Huang, Carbohydr. Polym., 2015, 132, 351–358 CrossRef CAS PubMed
.
- K. Liu, L. Chen, L. Huang, Y. Ni and B. Sun, Carbohydr. Polym., 2015, 117, 996–1001 CrossRef CAS PubMed
.
- E. Saarikoski, M. Rissanen and J. Seppala, Carbohydr. Polym., 2015, 119, 62–70 CrossRef CAS PubMed
.
- J. You, J. Zhou, Q. Li and L. Zhang, Langmuir, 2012, 28, 4965–4973 CrossRef CAS PubMed
.
- H. Qian, X. Wang, K. Yuan, C. Xie, W. Wu, X. Jiang and L. Hu, Biomater. Sci., 2014, 2, 220–232 RSC
.
- H. Hu, L. Xing, Y. Hu, C. Qiao, T. Wu, G. Zhou and W. Zhang, Food Hydrocolloids, 2016, 52, 38–46 CrossRef CAS
.
- B. Ma, M. Zhang, C. He and J. Sun, Carbohydr. Polym., 2012, 88, 347–351 CrossRef CAS
.
- M. I. Vazquez, M. Algarra and J. Benavente, Carbohydr. Polym., 2015, 131, 273–279 CrossRef CAS PubMed
.
- M. Soheilmoghaddam, G. Sharifzadeh, R. H. Pour, M. U. Wahit, W. T. Whye and X. Y. Lee, Mater. Lett., 2014, 135, 210–213 CrossRef CAS
.
- L. Tang, X. Li, D. Du and C. He, Prog. Nat. Sci., 2012, 22, 341–346 CrossRef
.
- A. A. Taha, Y. N. Wu, H. Wang and F. Li, J. Environ. Manage., 2012, 112, 10–16 CrossRef CAS PubMed
.
- R. Li, L. Zhang and M. Xu, Carbohydr. Polym., 2012, 87, 95–100 CrossRef CAS
.
- Y. Feng, X. Zhang, Y. Shen, K. Yoshino and W. Feng, Carbohydr. Polym., 2012, 87, 644–649 CrossRef CAS
.
- C. Wan and J. Li, Mater. Des., 2015, 83, 620–625 CrossRef CAS
.
- S. M. L. Rosa, N. Rehman, M. I. G. de Miranda, S. M. B. Nachtigall and C. I. D. Bica, Carbohydr. Polym., 2012, 87, 1131–1138 CrossRef CAS
.
- C. Bilbao-Sainz, B. S. Chiou, D. Valenzuela Medina, W. X. Du, K. S. Gregorski, T. G. Williams, D. F. Wood, G. M. Glenn and W. J. Orts, Eur. Polym. J., 2014, 54, 1–10 CrossRef CAS
.
- E.-R. Jo, P.-M. Jung, J.-i. Choi and J.-W. Lee, Radiat. Phys. Chem., 2012, 81, 1259–1262 CrossRef CAS
.
- A. R. Fajardo, L. C. Lopes, A. O. Caleare, E. A. Britta, C. V. Nakamura, A. F. Rubira and E. C. Muniz, Mater. Sci. Eng., C, 2013, 33, 588–595 CrossRef CAS PubMed
.
- S. Pramanik, R. Konwarh, N. Barua, A. K. Buragohain and N. Karak, Biomater. Sci., 2014, 2, 192–202 RSC
.
- S. Rahmanian, A. R. Suraya, B. Roshanravan, R. N. Othman, A. H. Nasser, R. Zahari and E. S. Zainudin, Mater. Des., 2015, 88, 227–235 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23409c |
| ‡ Equal contributors. |
| This journal is © The Royal Society of Chemistry 2016 |