Synthesis of novel N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine derivatives as inducers of apoptosis in MCF-7 breast cancer cells†
Manjunath G. Sunagara,
Supreet Gaonkara,
Santosh G. Sunagarb,
Narahari Deshapandea,
Ningaraddi S. Belavagia and
Imtiyaz Ahmed M. Khazi*a
aDepartment of Chemistry, Karnatak University, Dharwad 580003, Karnataka, India. E-mail: drimkorgchem@gmail.com; Fax: +91-836-2771275; Tel: +91-836-2215286 extn 29
bBio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: santosh.sunagar@gmail.com
First published on 12th January 2016
Abstract
A series of N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine derivatives (PP05–PP21) were prepared and evaluated for their anticancer activity against a panel of human cancer cell lines. Evaluation of results revealed that some of the synthesized compounds exhibited promising anticancer activity against the examined cancer cell lines. The structure–activity relationship (SAR) studies in the present work revealed that simple N-9 alkyl substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purines are potent anticancer agents. Among all the compounds, PP17 (9-sec-butyl-6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine) showed good inhibitory activity against MCF-7 cells. Cell cycle analysis of the compound suggested that induces G2/M phase arrest. Biochemical experiments showed that PP17 significantly induced MCF-7 cell apoptosis. Therefore, compound PP17 with a potent in vitro anticancer activity can serve as a promising lead compound for further study.
Introduction
Purine is the most widely distributed and abundantly available nitrogen heterocyclic system in nature. Both naturally occurring and synthetic purines have been found to exhibit a wide range of biological activities.1 The diversity of purine libraries against some biological targets has contributed to the development of new therapeutic agents. In particular, substituted purine derivatives are found in current application as antivirals, interferon inducers, antimycobacterials, CRH-R1 modulators and inhibitors of Hsp90, leukotriene A4 hydrolase, sulfotransferase, phosphodiesterases, and kinases.2,3 In addition to this specifically functionalised purines have been found to treat cancer, which is one of the most common diseases and a leading cause of human death in the world. Biological target oriented synthesis has been successful in the case of the purine skeleton, in view of the molecular diversity that can be introduced at C-2, C-6 and C-8. The resulting molecular libraries will bear a close structural resemblance to adenine, guanine and hypoxanthines. This is reflected in many purines being employed as clinically accepted drugs in cancer chemotherapy. Some of the purine based anticancer drugs approved by FDA are idelalisib, nelarabine, clofarabine, mercaptopurine (Fig. 1).4–8Purine based anticancer drugs are nucleoside analogs and they affect the intrinsic structural features of DNA, leading to stalled replication forks and chain termination.9,10 These drugs act by converting into free nucleosides resistant to deamination by the enzyme adenosine deaminase (ADA) or by disrupting nucleotide metabolism by incorporation into DNA or by inhibition of ribonucleotide reductase (RnR).11,12
In substituted purines C-6, N-9 are the most widely exploited anchoring points and they are commercially available.13,14 Substitution reactions of 6-halogenopurines with different nucleophiles, have been widely exploited in drug discovery.15 The SNAr reactions between 6-halogenopurine nucleosides with nitrogen, oxygen and sulphur nucleophiles is well established in the literature.16 Alkylation of purines with different alkyls halides results in the formation of regioisomeric mixtures of N-7 and N-9 alkylpurines but the desired N-9 compound is normally the major product. Besides the formation of N-9 as a major isomer, the N-7 isomers and other alkylation products are also observed.17
Purines are excellent scaffold for the synthesis of significant molecules in biology and the wide diversity of the chemistries that are possible with this heterocycle. Further, the phenyl piperazine and its analogues are the important building blocks in drug discovery, across a number of different therapeutic areas including anticancer therapy.18 In particular, the p-propoxyphenyl piperazine moiety having three rotating bonds and nucleophilic centres with conformational flexibility has been a pharmacophore in a number of biologically active compounds. These phenyl piperazine scaffolds are reported to be used for inhibiting tumor metastasis in cancers like liver cancer, mammary cancer, ovary cancer, gastric cancer, colon cancer, lung cancer or melanoma etc.19–21 Mechanistic studies of phenyl piperazine analogues have showed that they inhibit microtubule synthesis, cell cycle progression, angiogenesis, and destroy the tumor cells through induction of apoptosis.22,23 In the light of this, we have synthesised novel series of N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine derivatives. These molecules were evaluated for their cytotoxicity against selected human cancer cell lines, and were found to exhibit significant in vitro anticancer activity.
Result and discussion
Chemistry
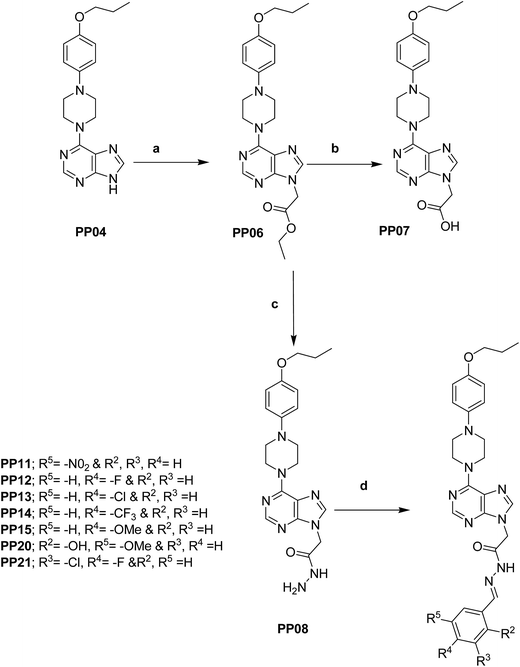
The synthesis of N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) was carried out as shown in Schemes 1 & 2. Commercially available 1-(4-(4-hydroxyphenyl)piperazin-1-yl)ethanone (PP01) was treated with 1-bromo propane in the presence of a base to obtain 1-(4-(4-propoxyphenyl)piperazin-1-yl)ethanone (PP02). This intermediate (PP02) was de-acetylated by using HCl (4 N) to obtain 1-(4-propoxyphenyl)piperazine (PP03). Finally, we achieved the synthesis of key intermediate 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) by reacting the intermediate PP03 with 6-chloro purine in presence of a base. The reaction of key intermediate (PP04) with different alkyl/aryl halides produced N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purines as shown in Scheme 2. | ||
| Scheme 1 Synthesis of 1-(4-propoxyphenyl)piperazine (PP03); reagents and conditions: (a) anhyd. K2CO3, DMF, 120 °C, 12 h; (b) 4 N HCl, 80 °C, 3 h. | ||
 | ||
| Scheme 2 Synthesis of N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purines; reagents and conditions: (a) triethyl amine (TEA), n-butanol, 110 °C, 12 h; (b) R1–Br, K2CO3, DMF, RT, 10 h. | ||
The key intermediate (PP04) obtained in the previous steps was treated with ethyl 2-bromoacetate in presence of base to afford ethyl 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetate (PP06), which on hydrolysis gave the acid 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetic acid (PP07). The intermediate (PP06) on treating with hydrazine hydrate gave the hydrazide (PP08). Finally, the hydrazide (PP08) reacted with different substituted aldehydes in ethanol to give the substituted (E)-N′-benzylidene-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazides as shown in Scheme 3.
Biology
| Compounds | % control growth (average value)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug concentrations (μg mL−1) | ||||||||||||
| HeLa | HCT-15 | MCF-7 | ||||||||||
| 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | |
| a Average values of triplicate experiments (n = 3). | ||||||||||||
| PP05 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 97.5 | 95.7 | 93.6 | 91.9 |
| PP06 | 96.8 | 94.5 | 92.7 | 90.7 | 100 | 100 | 100 | 100 | 99.1 | 98.0 | 95.1 | 93.3 |
| PP07 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 104 | 99.0 | 95.2 | 96.4 |
| PP08 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 93.7 | 89.9 | 91.6 |
| PP09 | 78.7 | 70.8 | 51.9 | 38.1 | 100 | 100 | 100 | 100 | 58.6 | 44.6 | 35.4 | 32.5 |
| PP10 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PP11 | 99.6 | 99.1 | 91.7 | 80.1 | 100 | 99.6 | 96.3 | 93.8 | 95.9 | 84.6 | 81.9 | 79.4 |
| PP12 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 101 | 92.9 | 92.7 | 98.1 |
| PP13 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 102 | 96.4 | 97.8 | 98.4 |
| PP14 | 100 | 100 | 100 | 100 | 100 | 100 | 98.9 | 99.1 | 97.6 | 94.3 | 93.7 | 95.4 |
| PP15 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.6 | 97.8 | 93.7 | 96.1 | 100.8 |
| PP16 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95.0 | 90.2 | 88.1 | 89.1 |
| PP17 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 97.4 | 35.0 | 31.0 | 27.5 | 29.8 |
| PP18 | 99.7 | 99.1 | 98.1 | 97.0 | 100 | 100 | 100 | 100 | 95.1 | 92.9 | 90.5 | 88.4 |
| PP19 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95.6 | 93.8 | 91.5 | 88.3 |
| PP20 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 88.9 | 86.7 | 83.4 | 81.2 |
| PP21 | 99.7 | 98.8 | 96.7 | 94.4 | 99.3 | 98.7 | 98.0 | 97.3 | 85.4 | 82.0 | 79.2 | 75.6 |
| ADR | −73.4 | −75.9 | −79 | −80.6 | −21.8 | −25.2 | −27.6 | −32.1 | −05.3 | −06.3 | −15.9 | −46.1 |
| Compounds | Drug concentrations calculated from graph (μg mL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HeLa | HCT-15 | MCF-7 | |||||||
| LC50 | TGI | GI50 | LC50 | TGI | GI50 | LC50 | TGI | GI50 | |
| a LC50 = concentration of drug causing 50% cell kill, GI50 = concentration of drug causing 50% inhibition of cell growth, TGI = concentration of drug causing total inhibition of cell growth, ADR = Adriamycin, positive control. | |||||||||
| PP05 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP06 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP07 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP08 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP09 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP10 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP11 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP12 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP13 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP14 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP15 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP16 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP17 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | <10 |
| PP18 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP19 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP20 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| PP21 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| ADR | 36.9 | <10 | <10 | 77.4 | 29.7 | <10 | 89.6 | <10 | <10 |
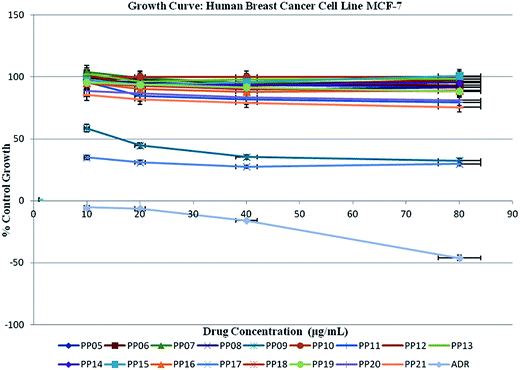
The structure–activity relationship (SAR) studies in the present investigation revealed that 4-propoxyphenyl piperazine moiety is essential for the optimal anticancer activity. This result prompted us to have variations at N-9 position keeping 4-propoxyphenyl piperazine constant at the C-6 position with a goal to optimize anticancer activity. The Schiff bases of acetohydrazides at N-9 position of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP11–15, PP20–21) displayed no activity in terms of both GI50 and % control growth, while the replacement of Schiff bases at N-9 with one carbon spacer carbonyl attached to aryl and hetero-aryl groups (PP05, PP10, PP16 & PP18–19) resulted in the moderate activity compared to standard Adriamycin.
Finally, the replacement at N-9 by simple alkyl chains (PP09 & PP17) afforded the molecule with potent anticancer activity. Interestingly, compound PP17 having iso butyl group at N-9 of purine showed good % control growth ranging from 29–35% with GI50 < 10 μg mL−1. Further introduction of larger alkyl chains such as 2-ethyl hexyl resulted in the reduction of activity.
Conclusions
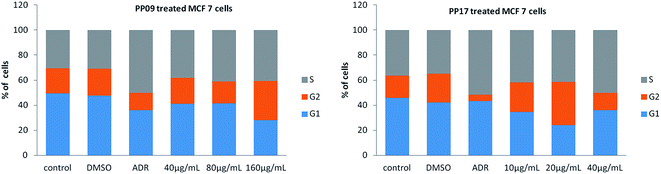
In summary, we have synthesised a series of novel N-9 substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine derivatives and tested for their in vitro anticancer activities against different human cancer cell lines. Among all the synthesised compounds only the compounds substituted with simple alkyl groups at N-9 position of purine moiety (PP09 and PP17) have shown selective anticancer activity in MCF-7 cell line. The SAR investigations of present work disclosed that simple N-9 alkyl substituted 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purines are potent anticancer agents than bulky N-9 substituted groups. Flow cytometric study results revealed that compounds PP09 and PP17 caused cell cycle arrest in the G2/M phase. Moreover, p53 expression results suggest that they caused cell death by inducing apoptosis. Based on these results, it is evident that PP17 can serve as a lead compound for further study.Experimental
Chemistry
Preparation of 1-(4-(4-propoxyphenyl)piperazin-1-yl)ethanone (PP02). To a solution of 1-(4-(4-hydroxyphenyl)piperazin-1-yl)ethanone (PP01) (5.0 g, 22.6 mmol) in dry DMF was added anhyd. K2CO3 (6.24 g, 45.2 mmol) under N2 atmosphere and stirred for 10–15 min at room temperature. Then 1-bromo propane (3.33 g, 27.10 mmol) was added drop wise and stirred at 70 °C for 15 min. Further, the reaction mixture was refluxed at 120 °C for 12 h. After completion of the reaction (checked by TLC), the reaction mixture was cooled to room temperature. The un-reacted K2CO3 was filtered off and washed with little amount of DMF. The DMF was evaporated under vacuum to obtain crude solid, which on washing with cold water yielded pure 1-(4-(4-propoxyphenyl)piperazin-1-yl)ethanone (PP02).
Yield 92%. Brown solid. Mp 118–120 °C, 1H NMR (400 MHz, CDCl3): δH 1.0 (3H, t, J = 16.0 Hz), 1.65–1.80 (2H, m), 2.12 (3H, s), 3.86 (2H, t, J = 12.0 Hz), 3.06 (4H, s), 3.75 (4H, s), 6.71 (2H, d, J = 7.5 Hz), 6.82 (2H, d, J = 7.5 Hz), 13C NMR (100 MHz, CDCl3): 10.2, 22.1, 23.5, 46.5, 51.0, 72.1, 113.4, 115.8, 145.4, 148.5, 170.1; LC/MS (ESI): M + 1 (C15H22N2O2), found 263.15, calculated 263.16.
Preparation of 1-(4-propoxyphenyl)piperazine (PP03). 1-(4-(4-Propoxyphenyl)piperazin-1-yl)ethanone (PP02) (4.0 g, 15.2 mmol) was dissolved in distilled water (15–20 mL). To this solution HCl (4 N) was added under N2 atmosphere. The mixture was stirred for 3 h at 80 °C and progress of reaction was monitored by TLC. The reaction mixture was cooled to 5–10 °C and neutralised with aq. NaOH (25%) to obtain solid compound. It was recrystallized from ethyl acetate to offer solid product 1-(4-propoxyphenyl)piperazine (PP03), which was taken as such for the next step.
Yield 89%. Pale white solid. Mp 154–156 °C, 1H NMR (400 MHz, CDCl3): δH 1.0 (3H, t, J = 16.0 Hz), 1.65–1.80 (2H, m), 2.2 (1H, s), 3.86 (2H, t, J = 12.0 Hz), 3.06 (4H, s), 3.75 (4H, s), 6.77 (2H, d, J = 7.8 Hz), 6.94 (2H, d, J = 7.8 Hz); 13C NMR (100 MHz, CDCl3): 10.47, 22.65, 46.3, 50.88, 69.88, 115.22, 118.90, 144.99, 154.0; LC/MS (ESI): M + 1 (C13H20N2O) found 221.15, calculated 221.17.
Preparation of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04). To 1-(4-propoxyphenyl)piperazine (PP03) (4.7 g, 21.35 mmol) obtained in the earlier step was added 6-chloro purine (3.0 g, 19.40 mmol) in n-butanol and, after 5 minutes triethylamine (5.87 g, 58.20 mmol) was added drop wise. After the completion of addition, stirring was continued at 110 °C for 10 h. Upon completion of the reaction n-butanol was removed under vacuum and the residual solid was recrystallized from ethanol to obtain pure 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) in good yields.
Yield 90%. White solid. Mp 230–232 °C, 1H NMR (400 MHz, DMSO-d6): δH 0.94 (3H, t, J = 7.6 Hz), 1.63–1.72 (2H, m), 3.10 (4H, s), 3.83 (2H, t, J = 6.4 Hz), 4.35 (4H, s), 6.81 (2H, d, J = 8.0 Hz), 6.92 (2H, d, J = 8.0 Hz), 8.14 (1H, s), 8.22 (1H, s), 13.06 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.8, 22.1, 48.3, 72.1, 115.6, 117.6, 119.3, 140.3, 144.4, 146.7, 152.1, 153.2, 154.5; LC/MS (ESI): M − 1 (C18H22N6O) found 337.40, calculated 337.43.
1-Phenyl-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)ethanone (PP05). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (300 mg, 0.886 mmol), 2-bromo-1-phenylethanone (212 mg, 1.063 mmol) and K2CO3 (366.9 mg, 2.659 mmol) in dry DMF (10 mL) as a brownish white coloured solid, mp 176–178 °C, (360.21 mg, yield 89.11%); 1H NMR (400 MHz, DMSO-d6): δH 0.94 (3H, t, J = 7.6 Hz), 1.63–1.72 (2H, m), 3.10 (4H, s), 3.83 (2H, t, J = 6.4 Hz), 4.35 (4H, s), 4.8 (2H, s), 6.81 (2H, d, J = 8.0 Hz), 6.92 (2H, d, J = 8.0 Hz), 7.68 (2H, d, J = 8.0 Hz), 7.20–7.29 (3H, m), 8.14 (1H, s), 8.22 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.2, 21.1, 49.3, 62.1, 72.0, 92.3, 114.6, 117.7, 119.8, 127.1, 128.5, 133.0, 137.5, 139.4, 144.4, 146.9, 152.5, 153.7, 155.5, LC/MS (ESI): M + 1 (C26H28N6O2) found 456.29, calculated 456.23.
Ethyl 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetate (PP06). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (300 mg, 0.886 mmol), ethyl 2-bromoacetate (177.64 mg, 1.063 mmol) and K2CO3 (366.9 mg, 2.659 mmol) in dry DMF (10 mL) as a white coloured solid, mp 148–150 °C, (336.71 mg, yield 89.58%); 1H NMR (400 MHz, DMSO-d6): δH 0.98 (3H, t, J = 3.6), 1.19–1.23 (3H, m), 1.65–1.73 (2H, m), 3.12–3.16 (4H, m), 3.85 (2H, t, J = 4.0 Hz), 4.14–4.20 (2H, m), 4.34–4.36 (4H, m), 5.10 (2H, s), 6.82–6.96 (4H, m), 8.20 (1H, s), 8.26 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.8, 14.6, 22.1, 48.3, 56.9, 61.2, 72.1, 115.6, 117.6, 119.3, 140.3, 144.4, 146.7, 152.1, 153.2, 154.5, 166.9; LC/MS (ESI): M − 1 (C22H28N6O3) found 424.26, calculated 424.23.
9-(2-Ethylhexyl)-6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP09). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (400 mg, 1.181 mmol), 3-(bromomethyl)heptanes (342.11 mg, 1.771 mmol) and K2CO3 (488.93 mg, 3.543 mmol) in dry DMF (10 mL) as a brown coloured solid, mp 90–92 °C, (457.64 mg, yield 86.05%); 1H NMR (400 MHz, CDCl3): δH 0.86 (3H, t, J = 6.45), 0.91 (3H, t, J = 6.48 Hz), 0.94 (3H, t, J = 7.6 Hz), 1.10–1.16 (2H, m), 1.20–1.25 (2H, m), 1.27–1.32 (2H, m), 1.34–1.39 (2H, m), 1.50–1.56 (1H, m), 1.63–1.72 (2H, m), 3.10 (4H, s), 3.83 (2H, t, J = 6.4 Hz), 3.90 (2H, d, J = 7.24 Hz), 4.35 (4H, s), 6.81 (2H, d, J = 8.0 Hz), 6.92 (2H, d, J = 8.0 Hz), 8.14 (1H, s), 8.22 (1H, s), 13C NMR (100 MHz, CDCl3): 10.8, 11.6, 14.2, 22.1, 23.5, 26.4, 29.1, 32.3, 36.9, 48.3, 57.5, 72.1, 115.6, 117.6, 119.3, 141.3, 143.4, 148.7, 152.1, 152.2, 154.5; LC/MS (ESI): M − 1 (C26H38N6O) found 450.30, calculated 450.32.
9-(4-Iodobenzyl)-6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP10). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (300 mg, 0.886 mmol), 1-(bromomethyl)-4-iodobenzene (315.86 mg, 1.063 mmol) and K2CO3 (366.9 g, 2.659 mmol) in dry DMF (10 mL) as a brown coloured solid, mp 160–162 °C, (402.30 mg, yield 81.94%); 1H NMR (400 MHz, DMSO-d6): δH 0.99 (3H, t, J = 7.5 Hz), 1.67–1.77 (2H, m), 3.16 (4H, s), 3.88 (2H, t, J = 6.6 Hz), 4.37 (4H, s), 5.25 (2H, s), 6.78 (2H, d, J = 8.0 Hz), 6.96 (2H, d, J = 8.0 Hz), 7.36 (2H, d, J = 5.49 Hz), 7.58 (2H, d, J = 8.29 Hz), 8.26 (1H, s), 8.37 (1H, s), 13C NMR (100 MHz, DMSO-d6): 11.1, 21.1, 49.3, 54.6, 74.1, 91.6, 115.2, 118.4, 120.5, 130.5, 135.5, 137.8, 141.3, 143.5, 147.5, 151.1, 153.1, 155.5; LC/MS (ESI): M + 1 (C25H27IN6O) found 554.11, calculated 554.14.
7-Methyl-4-((6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)methyl)-2H-chromen-2-one (PP16). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (400 mg, 1.182 mmol), 4-(bromomethyl)-7-methyl-2H-chromen-2-one (327.83 mg, 1.301 mmol) and K2CO3 (358.30 g, 3.548 mmol) in dry DMF (10 mL) as a light yellow coloured solid, mp 206–208 °C, (504.0 mg, yield 83.07%); 1H NMR (400 MHz, CDCl3): δH 0.90 (3H, t, J = 7.6 Hz), 1.63–1.72 (2H, m), 2.36 (3H, s), 3.22 (4H, s), 3.83 (2H, t, J = 6.4 Hz), 3.90 (4H, s), 5.22 (2H, s), 5.93 (1H, s), 6.81 (2H, d, J = 8.0 Hz), 6.92 (2H, d, J = 8.0 Hz), 7.14 (1H, s), 7.58 (1H, d, J = 7.8 Hz), 7.66 (1H, d, J = 7.8 Hz), 8.14 (1H, s), 8.22 (1H, s), 13C NMR (100 MHz, CDCl3): 10.8, 21.41, 22.1, 48.3, 51.67, 72.1, 113.50, 114.71, 115.6, 116.82, 117.6, 119.3, 128.55, 129.23, 137.0, 139.35, 140.84, 144.4, 146.7, 152.1, 153.2, 154.5, 155.80, 159.65; LC/MS (ESI): M + 1 (C29H30N6O3) found 510.26, calculated 510.24.
9-sec-Butyl-6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP17). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (400 mg, 1.1828 mmol), 2-bromobutane (178.27 mg, 1.3010 mmol) and K2CO3 (358.38 mg, 3.5484 mmol) in dry DMF (10 mL) as a pale white coloured solid, mp 84–86 °C, (410.26 mg, yield 88.03%); 1H NMR (400 MHz, CDCl3): δH 0.94 (3H, t, J = 7.6 Hz), 1.08 (3H, t, J = 6.68 Hz), 1.48 (3H, d, J = 7.11 Hz), 1.63–1.72 (2H, m), 1.94–1.99 (2H, m), 3.10 (4H, s), 3.83 (2H, t, J = 6.4 Hz), 4.35 (4H, s), 4.58 (1H, m), 6.81 (2H, d, J = 8.0 Hz), 6.92 (2H, d, J = 8.0 Hz), 8.14 (1H, s), 8.22 (1H, s), 13C NMR (100 MHz, CDCl3): 9.3, 10.7, 22.0, 22.6, 32.5, 48.3, 54.1, 72.5, 115.6, 117.9, 119.7, 141.3, 144.4, 146.7, 152.1, 153.2, 154.5; LC/MS (ESI): M + 1 (C22H30N6O) found 394.26, calculated 394.23.
1-(4-Chlorophenyl)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)ethanone (PP18). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (250 mg, 0.7387 mmol), 2-bromo-1-(4-chlorophenyl)ethanone (205.59 mg, 0.8864 mmol) and K2CO3 (305.82 mg, 2.2161 mmol) in dry DMF (10 mL) as a pale yellow coloured solid, mp 196–198 °C, (305.20 mg, yield 84.28%); 1H NMR (400 MHz, DMSO-d6): δH 0.97 (3H, t, J = 7.6 Hz), 1.65–1.73 (2H, m), 3.14 (4H, s), 3.86 (2H, t, J = 6.4 Hz), 4.38 (4H, s), 5.18 (2H, s), 6.89 (2H, d, J = 8.0 Hz), 6.98 (2H, d, J = 8.0 Hz), 7.53 (2H, d, J = 8.76 Hz), 7.86 (2H, d, J = 8.67 Hz), 8.26 (1H, s), 8.37 (1H, s), 13C NMR (100 MHz, DMSO-d6): 11.2, 21.1, 48.6, 61.7, 72.6, 115.5, 117.3, 119.5, 128.9, 130.5, 135.3, 138.5, 141.3, 144.4, 146.8, 152.3, 153.1, 154.2, 192.3; LC/MS (ESI): M + 1 (C26H27ClN6O2) found 490.14, calculated 490.17.
2-(6-(4-(4-Propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)-1-(4-(trifluoromethoxy)phenyl)ethanone (PP19). It was prepared by following the above method by the addition of 6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purine (PP04) (250 mg, 0.7387 mmol), 2-bromo-1-(4-(trifluoromethoxy)phenyl)ethanone (249.90 mg, 0.8864 mmol) and K2CO3 (305.82 mg, 2.2161 mmol) in dry DMF (10 mL) as a white coloured solid, mp 212–214 °C, (326.84 mg, yield 81.90%); 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.6 Hz), 1.64–1.75 (2H, m), 3.13 (4H, s), 3.87 (2H, t, J = 6.4 Hz), 4.35 (4H, s), 5.24 (2H, s), 6.86 (2H, d, J = 8.0 Hz), 6.95 (2H, d, J = 8.0 Hz), 7.44 (2H, d, J = 8.45 Hz), 7.98 (2H, d, J = 8.45 Hz), 8.26 (1H, s), 8.37 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.6, 21.9, 49.3, 60.9, 71.1, 114.5, 115.8, 117.3, 119.6, 121.6, 129.1, 129.8, 141.3, 144.6, 146.4, 151.9, 153.5, 154.2, 166.4, 190.8; LC/MS (ESI): M + 1 (C27H27F3N6O3) found 540.26, calculated 540.23.
Preparation of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetic acid (PP07). To a stirred solution of ethyl 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetate (PP06) (500 mg, 1.177 mmol) in water (5 mL), aq. NaOH (2 M) was added and the mixture was stirred at 70–80 °C for 2–3 h. The reaction mixture was neutralized with HCl (3 M) till the pH = 3.0. The resulting solution was extracted with ethyl acetate and the combined organic layers were dried over anhydrous sodium sulfate. After concentration in vacuo it yielded pure 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetic acid (PP07).
Yield 86%. Pale white solid. Mp 240–242 °C, 1H NMR (400 MHz, CDCl3): δH 0.99 (3H, t, J = 7.6 Hz), 1.67–1.78 (2H, m), 3.16 (4H, s), 3.87 (2H, t, J = 6.4 Hz), 4.38 (4H, s), 4.78 (2H, s), 6.87 (2H, d, J = 8.0 Hz), 6.95 (2H, d, J = 8.0 Hz), 8.12 (1H, s), 8.58 (1H, s), 11.4 (1H, s), 13C NMR (100 MHz, CDCl3): 10.1, 21.7, 49.3, 59.6, 72.9, 115.5, 117.9, 119.8, 141.5, 144.5, 146.9, 152.1, 153.1, 154.5, 176.2; LC/MS (ESI): M − 1 (C20H24N6O3) found 396.19, calculated 396.17.
Preparation of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08). To a solution of ethyl 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetate (PP06) (500 mg, 1.178 mmol) in ethanol (10 mL) at RT, hydrazine hydrate was added (176.89 mg, 3.533 mmol) and resulting solution was stirred at reflux temperature for 4 h. It was then cooled to room temperature and ethanol was removed under high vacuo and the resulting compound was recrystallized from ethanol to obtain pure 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08).
Yield 91%. White solid. Mp 232–234 °C, 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.2 Hz), 1.66–1.71 (2H, m), 2.0 (2H, s), 3.13 (4H, s), 3.84 (2H, t, J = 6.0 Hz), 4.35 (2H, s), 4.83 (4H, s), 6.82 (2H, d, J = 8.4 Hz), 6.94 (2H, d, J = 8.4 Hz), 8.15 (1H, s), 8.23 (1H, s), 9.46 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.8, 21.7, 40.5, 49.3, 49.8, 71.6, 114.5, 116.3, 119.4, 140.8, 141.5, 147.8, 150.0, 152.6, 154.8, 165.5; LC/MS (ESI): M + 1 (C20H26N8O2) found 410.24, calculated 410.23.
(E)-N′-(3-Nitrobenzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP11). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (200 mg, 0.4878 mmol) and 3-nitro benzaldehyde (110.57 mg, 0.7317 mmol) in absolute ethanol (20 mL) as a white coloured solid, mp 204–206 °C, (201.36 mg, yield 75.99%); 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.2 Hz), 1.66–1.71 (2H, m), 3.13 (4H, s), 3.84 (2H, t, J = 6.0 Hz), 4.35 (2H, s), 4.83 (4H, s), 6.82 (2H, d, J = 8.4 Hz), 6.94 (2H, d, J = 8.4 Hz), 7.58–7.64 (2H, m), 8.05 (1H, s), 8.15 (1H, s), 8.23 (1H, s), 8.30 (1H, m), 8.65 (1H, s), 9.46 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.3, 22.7, 39.5, 49.1, 49.4, 71.7, 114.5, 115.3, 119.9, 122.9, 124.6, 130.1, 134.3, 136.4, 140.8, 141.9, 143.4, 146.8, 150.4, 152.6, 153.8, 170.5; LC/MS (ESI): M + 1 (C27H29N9O4) found 543.26, calculated 543.24.
(E)-N′-(4-Fluorobenzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP12). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (250 mg, 0.6097 mmol) and 4-flouro benzaldehyde (151.36 mg, 1.2195 mmol) in absolute ethanol (20 mL) as a white coloured solid, mp 244–246 °C, (249.80 mg, yield 79.36%); 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.2 Hz), 1.66–1.71 (2H, m), 3.13 (4H, s), 3.84 (2H, t, J = 6.0 Hz), 4.35 (2H, s), 4.83 (4H, s), 6.87 (1H, s), 6.90 (2H, d, J = 8.4 Hz), 7.15 (1H, s), 7.80 (2H, d, J = 8.4 Hz), 7.98 (2H, d, J = 8.71 Hz), 8.22 (2H, d, J = 8.71 Hz), 8.50 (1H, s), 9.98 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.4, 22.6, 39.8, 69.8, 114.8, 115.2, 118.9, 129.5, 130.9, 140.7, 141.1, 143.5, 147.7, 150.1, 152.5, 154.8, 165.5, 173.4, LC/MS (ESI): M + 1 (C27H29FN8O2) found 516.80, calculated 516.82.
(E)-N′-(4-Chlorobenzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP13). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (250 mg, 0.6097 mmol) and 4-chloro benzaldehyde (128.57 mg, 0.9146 mmol) in absolute ethanol (20 mL) as a white coloured solid, mp 238–240 °C, (270.37 mg, yield 83.32%); 1H NMR (400 MHz, DMSO-d6): δH 0.95 (3H, t, J = 7.3 Hz), 1.65–1.71 (2H, m), 3.14 (4H, s), 3.86 (2H, t, J = 6.0 Hz), 4.37 (2H, s), 4.86 (4H, s), 6.85 (1H, s), 7.12 (1H, s), 7.80 (2H, d, J = 8.4 Hz), 7.90 (2H, d, J = 8.4 Hz), 7.98 (2H, d, J = 8.71 Hz), 8.22 (2H, d, J = 8.71 Hz), 8.42 (1H, s), 9.88 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.6, 22.5, 40.1, 71.9, 114.9, 115.4, 116.0, 119.5, 129.6, 130.8, 140.5, 141.3, 143.6, 147.7, 150.5, 152.6, 154.9, 165.5, 173.6, LC/MS (ESI): M + 1 (C27H29ClN8O2) found 532.19, calculated 532.21.
(E)-N′-(4-(Trifluoromethyl)benzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP14). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (220 mg, 0.5365 mmol) and 4-trifluoromethyl benzaldehyde (140.12 mg, 0.8049 mmol) in absolute ethanol (20 mL) as a light yellow coloured solid, mp 250–252 °C, (248.36 mg, yield 81.75%); 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.2 Hz), 1.67–1.72 (2H, m), 3.22 (4H, s), 3.87 (2H, t, J = 6.4 Hz), 4.39 (2H, s), 5.10 (4H, s), 6.88 (1H, s), 7.05 (1H, s), 7.81 (2H, d, J = 7.6 Hz), 7.91 (2H, d, J = 8.0 Hz), 7.98 (2H, d, J = 8.0 Hz), 8.23 (2H, d, J = 6.0 Hz), 8.40 (1H, s), 9.99 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.8, 22.7, 40.5, 72.0, 114.6, 115.5, 116.3, 119.6, 129.7, 131.0, 140.4, 141.6, 143.8, 147.8, 150.4, 152.7, 155.0, 165.7, 173.8, LC/MS (ESI): M − 1 (C28H29F3N8O2) found 566.20, calculated 566.20.
(E)-N′-(4-Methoxybenzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP15). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (250 mg, 0.6097 mmol) and 4-methoxybenzaldehyde (124.43 mg, 0.9146 mmol) in absolute ethanol (20 mL) as a white coloured solid, mp 256–258 °C, (263.14 mg, yield 81.80%); 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.3 Hz), 1.66–1.72 (2H, m), 3.15 (4H, s), 3.66 (3H, s), 3.87 (2H, t, J = 6.0 Hz), 4.39 (2H, s), 4.86 (4H, s), 6.87 (1H, s), 7.14 (1H, s), 7.81 (2H, d, J = 8.4 Hz), 7.91 (2H, d, J = 8.71 Hz), 7.99 (2H, d, J = 8.4 Hz), 8.23 (2H, d, J = 8.71 Hz), 8.42 (1H, s), 9.88 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.8, 22.7, 40.6, 55.9, 72.0, 114.8, 115.5, 116.5, 119.4, 129.8, 130.7, 140.6, 141.5, 143.7, 147.5, 150.6, 152.3, 155.0, 165.7, 173.5, LC/MS (ESI): M + 1 (C28H32N8O3) found 528.24, calculated 528.26.
(E)-N′-(2-Hydroxy-5-methoxybenzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP20). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (350 mg, 0.8536 mmol) and 2-hydroxy-5-methoxybenzaldehyde (194.69 mg, 1.2804 mmol) in absolute ethanol (20 mL) as a light yellow coloured solid, mp 266–268 °C, (385.10 mg, yield 82.89%); 1H NMR (400 MHz, DMSO-d6): δH 0.95 (3H, t, J = 7.3 Hz), 1.66–1.72 (2H, m), 3.14 (4H, s), 3.74 (3H, s), 3.86 (2H, t, J = 6.0 Hz), 4.40 (2H, s), 4.87 (4H, s), 5.13 (1H, s), 6.83 (2H, d, J = 8.4 Hz), 6.92 (2H, d, J = 8.4 Hz), 7.05 (1H, s), 6.98 (2H, d, J = 8.71 Hz), 6.85 (1H, s), 7.15 (1H, s), 8.27 (1H, s), 9.51 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.6, 22.8, 40.7, 55.9, 72.1, 114.7, 115.6, 116.5, 119.4, 129.7, 130.7, 140.8, 141.5, 143.8, 147.5, 150.8, 152.5, 155.0, 165.7, 173.6, LC/MS (ESI): M + 1 (C28H32N8O4) found 544.28, calculated 544.25.
(E)-N′-(3-Chloro-4-fluorobenzylidene)-2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP21). It was prepared by following the above method by the addition of 2-(6-(4-(4-propoxyphenyl)piperazin-1-yl)-9H-purin-9-yl)acetohydrazide (PP08) (350 mg, 0.8536 mmol) and 3-chloro-4-fluorobenzaldehyde (202.29 mg, 1.2804 mmol) in absolute ethanol (20 mL) as a white coloured solid, mp 210–212 °C, (370.10 mg, yield 78.80%); 1H NMR (400 MHz, DMSO-d6): δH 0.96 (3H, t, J = 7.2 Hz), 1.66–1.71 (2H, m), 3.15 (4H, s), 3.87 (2H, t, J = 6.0 Hz), 4.37 (2H, s), 4.84 (4H, s), 6.83 (2H, d, J = 8.4 Hz), 6.95 (2H, d, J = 8.4 Hz), 7.14 (2H, d, J = 8.71 Hz), 7.50 (1H, s), 8.12 (1H, s), 8.14 (1H, s), 8.24 (1H, s), 9.47 (1H, s), 13C NMR (100 MHz, DMSO-d6): 10.5, 22.7, 40.1, 71.7, 114.8, 115.2, 115.9, 119.4, 129.5, 130.9, 140.7, 141.1, 142.1, 147.6, 150.2, 152.5, 154.8, 165.4, 174.1, LC/MS (ESI): M + 1 (C27H28ClFN8O2) found 550.23, calculated 550.21.
SRB assay24,25
The cell lines were grown in RPMI 1640 medium containing 10% foetal bovine serum and 2 mM L-glutamine. Cells were inoculated into 96 well microtiter plates in 100 μL. After cell inoculation, the microtiter plates were incubated at 37 °C in 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of experimental drugs.Experimental drugs were initially solubilised in dimethyl sulfoxide at 100 mg mL−1 and diluted to 1 mg mL−1 using water and stored frozen prior to use. At the time of drug addition, an aliquot of frozen concentrate (1 mg mL−1) was thawed and diluted to 100 μg mL−1, 200 μg mL−1, 400 μg mL−1 and 800 μg mL−1 with complete medium containing test article. Aliquots of 10 μL of these different drug dilutions were added to the appropriate microtiter wells already containing 90 μL of medium, resulting in the required final drug concentrations i.e. 10 μg mL−1, 20 μg mL−1, 40 μg mL−1, 80 μg mL−1.
After compound addition, plates were incubated at standard conditions for 48 h and assay was terminated by the addition of cold TCA. Cells were fixed in situ by the gentle addition of 50 μL of cold 30% (w/v) TCA (final concentration, 10% TCA) and incubated for 60 min at 4 °C. The supernatant was discarded; the plates were washed five times with water and air dried. Sulforhodamine B (SRB) solution (50 μL) at 0.4% (w/v) in 1% acetic acid was added to each of the wells, and plates were incubated for 20 min at room temperature. After staining, unbound dye was recovered and the residual dye was removed by washing five times with 1% acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM trizma base, and the absorbance was read on plate reader at a wavelength of 540 nm with 690 nm reference wavelength.
Percent growth was calculated on a plate-by-plate basis for test wells relative to control wells. Percent growth was expressed as the ratio of average absorbance of the test well to the average absorbance of the control wells × 100. Using the six absorbance measurements the percentage growth was calculated at each of the drug concentration levels.
Cell cycle analysis
Breast cancer cells (MCF-7) were seeded on 35 mm dishes and cultured for 24 h, reaching 60–70% confluency. The cells were incubated for 24 h in the presence or absence of test compounds of PP09 & PP17, harvested by trypsinization, resuspended in 200 mL PBS, and fixed in 70% ethanol for 30 min at 4 °C. Cells were stained with 1 mL of DNA staining solution (100 μg of RNase A and 40 μg of propidium iodide (PI)) for 30 min. The DNA content was measured and assessed on a FACS Calibur Flow cytometer using Cell Quest Pro software (BD Bioscience).Western blotting analysis
After the treatment of MCF-7 cells with compounds PP09 & PP17 cells were washed with ice cold phosphate-buffered saline (PBS) and cells were homogenized and lysed in RIPA buffer [20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate, 1 mM Na3VO4] supplemented 1 mM PMSF, and 1 mg mL−1 of aprotinin, leupeptin, and pepstatin A. Protein concentration was determined using a Bio-Rad assay. Proteins were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were incubated with antibodies p53 (Abcam) and b-actin (Santa Cruz Biotechnology) in 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween-20.Acknowledgements
The authors gratefully acknowledge the financial assistance from Department of Science & Technology (DST-SERB), Govt. of India, New Delhi under Major Research Project No. SR/S1/OC-58/2011 and University Grant Commission (UGC), New Delhi under UPE-FAR-I program, F. No. 14-3/2012 (NS/PE).References
- H. Rosemeyer, Chem. Biodiversity, 2004, 1, 361–401 CAS.
- M. Legraverend and D. S. Grierson, Bioorg. Med. Chem., 2006, 14, 3987–4006 CrossRef CAS PubMed.
- N. K. Anand, C. M. Blazey, O. J. Bowles, J. Bussenius, L. C. Bannen, D. S. Chan, B. Chen, E. Wang Co, S. Costanzo, S. C. Defina, L. Dubenko, M. Franzini, P. Huang, V. Jammalamadaka, R. G. Khoury, M. H. Kim, R. R. Klein, D. T. Le, M. B. Mac, J. M. Nuss, J. J. Parks, K. D. Rice, T. H. Tsang, A. L. Tsuhako, Y. Wang and W. Xu, US Pat., US8076338, 2011.
- D. S. Sanford, W. G. Wierda, J. A. Burger, M. J. Keating and S. M. O'Brien, Clin. Lymphoma, Myeloma Leuk., 2015, 15(7), 385–391 CrossRef PubMed.
- C. Pui, S. Jeha and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2005, 4, 369–370 CrossRef CAS PubMed.
- V. Gandhi, M. J. Keating, G. Bate and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2006, 5, 17–18 CrossRef CAS PubMed.
- FDA Orange Book, http://www.accessdata.fda.gov/scripts/cder/ob/docs/queryai.cfm.
- W. B. Parker, Chem. Rev., 2009, 109(7), 2880–2893 CrossRef CAS PubMed.
- D. Sampath, V. A. Rao and W. Plunkett, Oncogene, 2003, 22, 9063–9074 CrossRef CAS PubMed.
- G. B. Elion, Science, 1989, 244(4900), 41–47 CAS.
- G. R. Goodman, E. Beutler and A. Saven, Best Pract. Res., Clin. Haematol., 2003, 16(1), 101–116 CrossRef CAS PubMed.
- S. Faderl, V. Gandhi, M. J. Keating, S. Jeha, W. Plunkett and H. M. Kantarjian, Cancer, 2005, 103(10), 1985–1995 CrossRef CAS PubMed.
- A. R. Kore, B. Yang and B. Srinivasan, Curr. Org. Chem., 2014, 18(16), 2072–2107 CrossRef CAS.
- D. Bliman, M. Pettersson, M. Bood and M. Grøtli, Tetrahedron Lett., 2014, 55, 2929–2931 CrossRef CAS.
- M. Legraverend, Tetrahedron, 2008, 64, 8585–8603 CrossRef CAS.
- J. Liu and M. J. Robins, J. Am. Chem. Soc., 2007, 129, 5962–5968 CrossRef CAS PubMed.
- M. Zhong and M. J. Robins, J. Org. Chem., 2006, 71, 8901–8906 CrossRef CAS PubMed.
- M. Yarim, M. Koksal, I. Durmaz and R. Atalay, Int. J. Mol. Sci., 2012, 13, 8071–8085 CrossRef CAS PubMed.
- J. Fengchao, D. Yulan and J. Jie, WO Pat., WO2012163291, 2012.
- K. Dong Gyu, S. Dong Su, J. Sin, H. Ji Yeong and L. Myeong Jin, KR Pat., KR2012119229, 2012.
- M. A. Williams, WO Pat., WO2012061337, 2012.
- A. Chopra, A. Anderson and C. Giardina, J. Biol. Chem., 2014, 289(5), 2978–2991 CrossRef CAS PubMed.
- J. Sun, X. Wang, P. Lv and H. Zhu, Sci. Rep., 2015, 5, 13934 CrossRef PubMed.
- V. Vichai and K. Kirtikara, Nat. Protoc., 2006, 1, 1112–1116 CrossRef CAS PubMed.
- P. Skehn, R. Storeng, A. Scudiero, J. Monks, D. McMohan, D. Vistica, T. W. Jonathan, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82(13), 1107–1112 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23242b |
| This journal is © The Royal Society of Chemistry 2016 |