Solvent strategy for unleashing the Lewis acidity of titanocene dichloride for rapid Mannich reactions†
Ya Wuab,
Xiu Wanga,
Yanlong Luoa,
Jing Wanga,
Yajun Jiana,
Huaming Suna,
Guofang Zhanga,
Weiqiang Zhang*a and
Ziwei Gao*a
aKey Laboratory of Applied Surface and Colloid Chemistry, MOE, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, China. E-mail: zwgao@snnu.edu.cn; Fax: +86-29-81530727
bCollege of Chemistry and Chemical Engineering, Xi'an Shiyou University, Xi'an 710065, P. R. China
First published on 21st January 2016
Abstract
The remarkable activation effect of alcohol solvent on kinetically inert titanocene dichloride was found to promote rapid three-component Mannich reactions. NMR and ESI-MS analyses as well as a control experiment of catalytic active species elucidated that the coordination of MeOH to the titanocene moiety unleashed the Lewis acid [Cp2Ti(OMe)2] and Brønsted acid HCl, which led to the enhanced catalytic activity of [Cp2TiCl2].
Organometallic Lewis acids have been of fundamental interest in homogenous catalysis due to their ubiquitous reactivity and selectivity in classic organic transformations.1 In organometallic Lewis acid catalysis the ligand and solvent are two critical factors determining the reaction efficiency.2 With the ligand effect having been clearly demonstrated to have a significant role in the catalytic reaction over the decades,3 sophisticated co-ligands are judiciously selected to stabilize Lewis acid counterparts and the involved ligand inevitably requires tedious multi-step syntheses.4 The other critical factor is that solvent can direct unprecedented chemical reactivity and selectivity in catalytic organic transformations owing to the fact that the solvents to be introduced would be able to adjust the steric balance, tune coordination of the metallic centre, and control hydrolysis and condensation.5 For instance, Brønsted acidic solvents provided a green and sustainable option for maximising the productivity of the zinc reagent catalysed Biginelli reaction.5c Using γ-valerolactone as the solvent led to significant increase in reaction rate as well as product selectivity in acid catalysis of xylose into furfural.6 In addition, it is much easier to investigate the solvent effect as various solvent are ready to be used. However, compared with the ligand effect, less attention is paid to the solvent effect, so detailed mechanistic information on how they affect reactions is still rare.
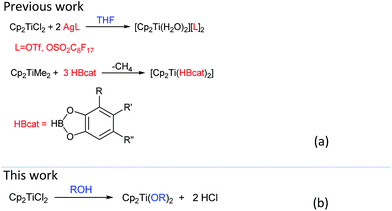
Group IVB metallocenes, especially, titanocenes have been proved to be promising Lewis acid catalyst precursors7 due to its kinetic stability, electronic tunable metal center and intrinsic metallic Lewis acidity.8 Usually, the titanocene Lewis acids were obtained by introducing weakly coordinating anion ligands or neutral ligands. For example, titanocene complexes [Cp2Ti(HBcat)2],9 Cp2Ti[P(OEt)3]2,10 [Ti(Cp)2(H2O)2(CF3SO3)2],11 and [Ti(Cp)2(H2O)2(C8F17SO3)2]12 as potential organometallic Lewis acid catalyst showed particularly activity for the transformation of carbonyl compounds (Scheme 1(a)). However, employment of expensive silver salts or moisture-sensitive reagents or low yield limited their further application in the activation of kinetically inert titanocene complexes via ligands strategy. Therefore, developing a solvent activation strategy for titanocene Lewis acids is still of high significance.
Recently, we have disclosed a type of titanocene complexes with increasing Lewis acidity by the cooperative effect of salicylic acid ligands. Our ongoing interest in titanocene Lewis acid catalysis promoted us to investigate more efficient activation strategies of the stable titanocene complexes.13 Considering facile substitution reactions of alkoxy, acyloxy and hydroxyl with Cp2TiCl2 in ethanol, THF and water,14 it was feasible to improve the activity of Cp2TiCl2 by the interaction of solvent with Ti moiety. The introduction of suitable solvent can be advantageous to obtain titanocene Lewis acid complexes. However, to the best of our knowledge, solvent effect hasn't been proposed to enhance catalytic activity of titanocene Lewis acids.
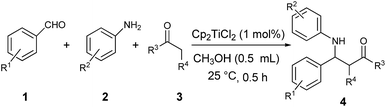
In this paper, we introduced a solvent strategy for unleashing Lewis acid and Brønsted acid via activating titanocene dichloride for rapid Mannich reactions (Scheme 1(b)). Kinetically inert titanocene dichloride showed a significant activation in alcohols and efficiently catalyzed three-component Mannich of aldehydes, ketones and amines. Mechanism study including NMR and ESI-MS analyses as well as control experiment shed light on the effect of alcohols on releasing Lewis acidity of titanocene dichloride along with Brønsted acidity, which led to the dramatic catalytic performances. To the best of our knowledge, this constitutes the first time that the intrinsic property of solvent dictates the activation of stable Cp2TiCl2 in catalytic organic transformation. Furthermore, the discovery provides a mild and efficient approach to conduct three-component Mannich reaction.
Mannich reactions have been developed as model reactions of evaluating solvent effect on the catalytic performance of titanocene dichloride because titanocene based catalyst was regarded to be sensitive to Mannich reaction according to our reported research.12,13 We began to investigate the Mannich reaction of benzaldehyde (1.0 mmol), aniline (1.1 mmol) and acetophenone (2.0 mmol) in the presence of titanocene dichloride (1 mol%) in diverse solvent (0.5 mL) at 25 °C. The results are demonstrated in Fig. 1. The catalytic activity of Cp2TiCl2 in the condensation reaction was greatly influenced by solvent. Preliminarily, traditionally used solvent CH2Cl2 was evaluated while Cp2TiCl2 was almost inert in CH2Cl2. Non-polar solvents such as benzene and n-hexane were not helpful for the Mannich reaction and gave only 18% and 6% yields after 0.5 h. THF and CHCl3 as polar solvent slightly accelerated the reaction (32% and 12% yields, respectively). More polar solvents such as DMSO and CH3CN were obviously active for Cp2TiCl2 catalysis. To our surprise, the condensation reaction accelerated dramatically when employing EtOH and furnished β-amino-carboxyl compound in 94% yield. Moreover, when MeOH replacing EtOH, the activity of Cp2TiCl2 was also enhanced slightly and the higher yield was obtained with 95%. The observations demonstrated that alcohol was a unique solvent and played an important role in the Cp2TiCl2 catalyzed Mannich reactions. Based on alcohols as a protic solvent, water was also paralleled to evaluate the reaction efficiency in the catalytic system. However, no product was detected. These results indicated that the catalytic activity of Cp2TiCl2 couldn't be contributed to solvent polarity or hydrogen-bonding interaction.
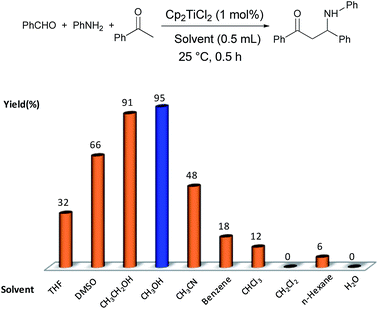 | ||
| Fig. 1 Solvent effect on titanocene dichloride catalyzed three-component reaction. aYield of isolated product. | ||
Various alcohols were further investigated to demonstrate the pronounced accelerating effect on titanocene catalyzed Mannich reactions (Table 1). In addition to MeOH and EtOH (entries 1 and 2), n-PrOH and n-BuOH featuring longer carbon-chain structure were also proved as a validated solvent (entries 3 and 5). Based on facile substitution reactions of alkoxy groups with Cp2TiCl2 in alcohols,14 it is probably because that the coordination between alcohols and titanocene species results in enhancing Lewis acidity of Ti centre and thus improving the catalytic efficiency. This hypothesis was further supported by the Mannich reactions catalysed by Cp2TiCl2 in sterically hindered alcohols such as i-PrOH, t-BuOH, PhCH2OH and cyclohexanol (entries 4, 6, 9 and 10). Owing to the fact that the sterical hindrance is disadvantageous to alcohols coordinating to organometallic centre, the yields of the condensation dramatically decreased and the reaction using t-BuOH only afforded 15% yield. Not surprisingly, it was also found that polyols i.e. ethylene glycol and glycerol suppressed the accelerating effect of short-chain monohydroxy alcohols, leading to only 28% and 7% yields of the Mannich reaction (entries 7 and 8) because titanocene dichloride in polyols readily formed a stable five-membered ring. On the other hand, the pKa of the alcoholic solvent was considered because it probably shed light on the propensity for acid catalysis. Despite low pKa of ethylene glycol and glycerol, the reaction failed to afford high yields. In a word, these results suggested it is a possibility that the coordination between alcohols and Ti center played a key role in unleashing Lewis acidity and improving catalytic activity of titanocene complexes. Besides, the optimized condition was also provided with 1 mol% titanocene catalyst and 0.5 mL MeOH solvent (entries 11 and 12).
| Entrya | Alcohol | pKa | Catalyst (mol%) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conducted with 1.0 mmol of benzaldehyde, 1.1 mmol of aniline, and 2.0 mmol of acetophenone in 0.5 mL alcohol at 25 °C for 0.5 h.b Isolated yields were obtained after purification by column chromatography. | ||||
| 1 | MeOH | 15.17 ± 0.10 | 1 | 95 |
| 2 | EtOH | 15.24 ± 0.10 | 1 | 94 |
| 3 | n-PrOH | 15.24 ± 0.10 | 1 | 88 |
| 4 | i-PrOH | 15.31 ± 0.20 | 1 | 79 |
| 5 | n-BuOH | 15.24 ± 0.10 | 1 | 83 |
| 6 | t-BuOH | 15.38 ± 0.29 | 1 | 15 |
| 7 | (CH2OH)2 | 14.13 ± 0.10 | 1 | 28 |
| 8 | Glycerol | 13.68 ± 0.20 | 1 | 7 |
| 9 | PhCH2OH | 14.36 ± 0.10 | 1 | 0 |
| 10 | C6H11OH | 15.31 ± 0.20 | 1 | 21 |
| 11 | MeOH | 15.17 ± 0.10 | 2 | 97 |
| 12 | MeOH | 15.17 ± 0.10 | 0.5 | 85 |
These findings led us to the discovery of a new protocol for inert titanocene dichloride by a solvent strategy accelerating the direct three-component Mannich reaction. The scope and limitation of the new solvent enhanced catalyst system were evaluated with a range of aldehydes, amines and aromatic and aliphatic ketones in the optimized conditions (Table 2). Substitutions on either anilines or benzaldehydes have little impact on the activity of the titanocene catalyst. Both electron-withdrawing and donating groups of aromatic substrates proved to be very effective, affording the Mannich condensation products in good yields (entries 1–12, 74–96%). Amine with chloro (4b) or methoxy (4c) substituents and aldehydes with chloro (4d) or methoxy (4e) substituents in para sites led to excellent results (85–96% yields) when acetophenone was used (entries 2–5). Moreover, the steric effect of substituents does not obviously interfere with the catalytic efficiency of the Mannich reaction. For instance, ortho-substituents 2-Cl and 2-OCH3 benzaldehyde (4f, 4l) and 2-Cl anilines (4k) condense with acetophenone with more than 70% yields (entries 6, 12 and 11).
| Entrya | R1 | R2 | R3, R4 | Yieldb (%) | anti/sync |
|---|---|---|---|---|---|
| a Reactions conducted with 1.0 mmol of aldehydes, 1.1 mmol of amines, and 2.0 mmol of ketones in the presence of 0.01 mmol of catalyst in 0.5 mL MeOH at 25 °C.b Isolated yields were obtained after purification by column chromatography.c Determined by 1H NMR analysis of crude products. | |||||
| 1 | H | H | Ph, H | 4a, 95 | n.d. |
| 2 | H | p-CH3 | Ph, H | 4b, 95 | n.d. |
| 3 | H | p-Cl | Ph, H | 4c, 85 | n.d. |
| 4 | p-OCH3 | H | Ph, H | 4d, 96 | n.d. |
| 5 | p-Cl | H | Ph, H | 4e, 88 | n.d. |
| 6 | o-Cl | H | Ph, H | 4f, 74 | n.d. |
| 7 | p-NO2 | H | Ph, H | 4g, 75 | n.d. |
| 8 | p-Cl | p-NO2 | Ph, H | 4h, 81 | n.d. |
| 9 | H | H | p-OCH3Ph, H | 4i, 90 | n.d. |
| 10 | H | H | p-NO2Ph, H | 4j, 81 | n.d. |
| 11 | H | o-Cl | Ph, H | 4k, 76 | n.d. |
| 12 | o-OCH3 | H | Ph, H | 4l, 85 | n.d. |
| 13 | H | p-CH3 | (CH2)4 | 4m, 88 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 14 | H | p-NO2 | (CH2)4 | 4n, 81 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
| 15 | p-Cl | H | (CH2)4 | 4o, 85 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
| 16 | p-NO2 | H | (CH2)4 | 4p, 72 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
| 17 | H | H | CH3, H | 4q, 71 | n.d. |
| 18 | p-Cl | H | CH3, H | 4r, 68 | n.d. |
| 19 | p-OCH3 | H | CH3, H | 4s, 79 | n.d. |
| 20 | H | H | C2H5, CH3 | 4t, 85 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
| 21 | H | p-OCH3 | C2H5, CH3 | 4u, 91 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 22 | H | p-Cl | C2H5, CH3 | 4v, 82 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 23 | p-Cl | H | C2H5, CH3 | 4w, 81 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 24 | p-OCH3 | p-Cl | C2H5, CH3 | 4x, 83 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
| 25 | H | H | n-C3H7, H | 4y, 77 | n.d. |
The alcohol accelerating [Cp2TiCl2] catalytic system was also successfully applied in the Mannich reactions of aliphatic ketones under optimized conditions (Table 2, entries 13–25). The reaction outcome was not significantly affected by aliphatic ketones. The catalytic Mannich condensation of cyclohexanone with substituted benzaldehydes and anilines afforded the products (4m–p) in 72–88% yields, whereas open chain ketones including acetone, 3-pentanone and 2-pentanone afforded the desired Mannich bases in 68–91% yield (4q–y). The strong electron-withdrawing groups such as nitro-substituted arylimine reacted much more slowly with a relatively low yield. To our delight, the catalytic system showed good anti-selectivity for the direct three-component Mannich reactions. As shown in Table 2, the catalytic Mannich condensation of 3-chloro-benzaldehyde, aniline and 3-pentanone afforded aliphatic aminoketone (4w) with 80/20 d.r. that was determined by 1H NMR spectroscopy.15 The corresponding reactions with substitutions on either anilines or benzaldehydes furnished the product with the values of anti/syn varied from 56/44 (4t) to 80/20 (4w) (entries 13–16 and 20–24).
To shed light on delicate accelerating effect of alcohols, the interaction between Cp2TiCl2 and MeOH was further investigated by 1H NMR and ESI(+)-MS, which were considered as powerful techniques to investigate the organometallic species.16 1H NMR experiments were conducted using Cp2TiCl2 in CD3OD at intervals with addition of aniline. The characteristic cyclopentadienyl (Cp) protons could be regarded as a probe to measure the formation of new titanocene complexes. In the 1H NMR spectrum only one prominent Cp singlet ( ) of Cp2TiCl2 (I) was detected at δ 6.64 ppm upon the addition of Cp2TiCl2 into CD3OD (Fig. 2), indicating no coordination happened. However, after 1.0 equiv. of aniline was added in the above solution, one new Cp protons singlet (
) of Cp2TiCl2 (I) was detected at δ 6.64 ppm upon the addition of Cp2TiCl2 into CD3OD (Fig. 2), indicating no coordination happened. However, after 1.0 equiv. of aniline was added in the above solution, one new Cp protons singlet ( ) appeared immediately at δ 6.70 ppm. Titanocene chloride (I) was consumed gradually in CD3OD in the presence of base and transformed to new titanocene species Cp2Ti(OCH3)2 (II) (Scheme 2), as revealed by ESI-MS analysis, corresponds to the [II + H]+ signal at m/z 241.0706. These observations suggest that the MeOH is not just a medium to dissolve the sandwich complexes but also a reactant involved in the process of activating Cp2TiCl2 via methoxy groups binding to Cp2TiIV moiety. So it is reasonable to draw a conclusion that in the Mannich reaction, pre-catalyst titanocene dichloride readily converted into the detectable titanocene species II, when methanol served as solvent. Comparing the chemical shifts of Cp protons of the Ti species, (I) and (II), the truth that the signal of (I) is downfiled shifted, indicating the Lewis acidity order as II > I. In addition, it was also found that II can readily decomposed as the cationic titanocene moiety [Cp2TiOCH3]+ (III) by releasing one methoxy ligand, verified by ESI(+)-MS spectrum (m/z 209.0445). The cation III was more electrophilic and should be originated from Cp2Ti(OCH3)2 (II).
) appeared immediately at δ 6.70 ppm. Titanocene chloride (I) was consumed gradually in CD3OD in the presence of base and transformed to new titanocene species Cp2Ti(OCH3)2 (II) (Scheme 2), as revealed by ESI-MS analysis, corresponds to the [II + H]+ signal at m/z 241.0706. These observations suggest that the MeOH is not just a medium to dissolve the sandwich complexes but also a reactant involved in the process of activating Cp2TiCl2 via methoxy groups binding to Cp2TiIV moiety. So it is reasonable to draw a conclusion that in the Mannich reaction, pre-catalyst titanocene dichloride readily converted into the detectable titanocene species II, when methanol served as solvent. Comparing the chemical shifts of Cp protons of the Ti species, (I) and (II), the truth that the signal of (I) is downfiled shifted, indicating the Lewis acidity order as II > I. In addition, it was also found that II can readily decomposed as the cationic titanocene moiety [Cp2TiOCH3]+ (III) by releasing one methoxy ligand, verified by ESI(+)-MS spectrum (m/z 209.0445). The cation III was more electrophilic and should be originated from Cp2Ti(OCH3)2 (II).
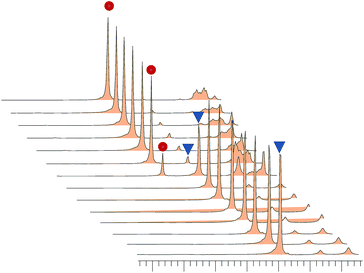 | ||
Fig. 2 Partial 400 MHz 1H NMR spectra (CD3OD) of a solution containing Cp2TiCl2 with addition of aniline.  6.64 ppm I [Cp2TiCl2]; 6.64 ppm I [Cp2TiCl2];  6.70 ppm II [Cp2Ti(OCH3)2]. 6.70 ppm II [Cp2Ti(OCH3)2]. | ||
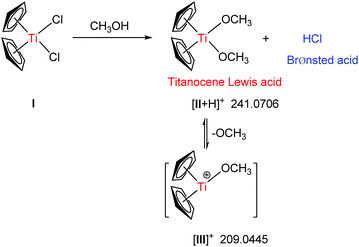 | ||
| Scheme 2 The coordination of Cp2TiCl2 with MeOH unleashed Lewis acidity and Brøsnted acidity by 1H NMR and ESI-MS. | ||
To further elucidate the catalysis mechanism and figure out the true catalysis species, a series of paralleled experiments were conducted under the same conditions as the original protocol. Above NMR and MS experiments show that Cp2Ti(OCH3)2 (II) was produced, and an equivalent amount of HCl should be released simultaneously. Although it is not easy to verify the existence of HCl directly, its formation can be rationalized by the NMR experiment that addition of organic base aniline assured the occurrence of methoxylation of Cp2TiCl2. Thus it is of great significance to shed light on the function of Cp2Ti(OCH3)2 (II) and HCl of the original reaction. As Brønsted acids are prevalent catalysts for Mannich reaction, HCl was primarily investigated, however, only 36% yield of product was isolated (Table 3, entry 2). Next, we evaluated the Cp2Ti(OCH3)2 (II) species, which was prepared following two literature procedures.17 We found that Cp2Ti(OCH3)2 could catalyze the reaction, but not as efficient as the original protocol, because the reaction yields are only 53% and 55% (Table 3, entries 3 and 4), respectively. However, adding a MeOH solution of HCl to entry 3, the condensation afforded an isolated yield of 93% (entry 5). It is clear that the high efficiency of the condensation shouldn't be attributed to Brønsted acid or titanocene Lewis acid sole. Conversely, we proposed that the co-involvement of the binary acids, Cp2Ti(OCH3)2 and HCl, resulted in the perfect coupling efficiency. This hypothesis are also in accordance with Yamamoto's binary acid principles.18 To sum up, the acceleration effect should result from the cooperative effect of Lewis acid and Brønsted acid released by Cp2TiCl2 in MeOH in situ.
| Entry | Catalyst (mol%) | Yieldb (%) |
|---|---|---|
| a Reaction conducted with 1.0 mmol of benzaldehyde, 1.1 mmol of aniline, and 2.0 mmol of acetophenone at 25 °C for 0.5 h.b Isolated yields were obtained after purification by column chromatography.c Without Cp2TiCl2 in 0.5 mL MeOH.d Formed in situ from Cp2TiCl2 (0.01 mmol) and Mg(OMe)2 (0.01 mmol) in MeOH (0.5 mL).e Obtained from a benzene solution of 1.0 equiv. Cp2TiCl2 with dropwise addition of a mixture of 2.0 equiv. MeOH and 2.0 equiv. triethylamine in benzene at 50 °C and removal of triethylamine hydrochloride by filtration. | ||
| 1c | — | 0 |
| 2 | HCl (2) | 36 |
| 3 | Cp2Ti(OCH3)2 (II) (1)d | 53 |
| 4 | Cp2Ti(OCH3)2 (II) (1)e | 55 |
| 5 | Cp2Ti(OCH3)2/HCl (1/2)d | 93 |
Taken NMR, MS and paralleled experiments together, a catalytic cycle via MeOH activation strategy for activating titanocene dichloride in Mannich reactions is proposed and listed in Scheme 3. Under the involvement of aniline, titanocene dichloride pre-catalyst is activated by MeOH to give titanocene dimethoxy complexes II and releases HCl simultaneously. The newly formed complex II is liable to release one methoxy ligand yielding the cation III which is prone to coordinate with ketone generates intermediate. In intermediate A, the enolization is accelerated synergistically as the carbonyl coordinate to oxophilic Ti and the leaving methoxy ligand abstracts the proton. The coordinated enolate then undergoes addition to the aldimine which is activated by protonation with the released HCl and Ti centre of III.19 The carbon–carbon bond formation as a key step is illustrated in 6-membered transition state B proposed in its chair conformation. This transition state B showcases the cooperative nature of this binary acid system unleashed by titanocene dichloride in MeOH. A critical examination of the diastereomeric outcome from the reactions corresponding to entries 13–16 and 20–24 in Table 2 should provide significant insight into the transition state for the C–C bond forming event because anti-isomers of the adducts compete favorably with syn-isomers.
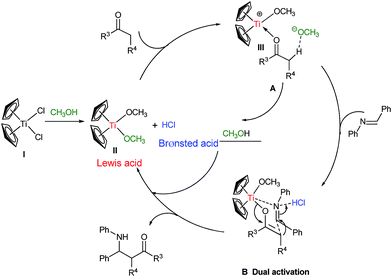 | ||
| Scheme 3 Proposed mechanism of binary acid catalysis by MeOH strategy of activating titanocene dichloride in Mannich reactions. | ||
Conclusions
In summary, a solvent strategy for improving inert organometallic complexes catalysis for rapid three-component Mannich reaction was developed. Mechanism study including NMR and ESI(+)-MS analyses as well as control experiment elucidated the effect of alcohols on the dramatic catalytic performances of titanocene dichloride for the condensation. The investigation of titanocene dichloride in MeOH has provided compelling evidence that coordination of MeOH to Cp2Ti moiety unleashed Lewis acid along with Brønsted acid, leading to cooperatively enhanced catalytic activity. Additionally, the new catalyst system allows for mild and rapid three-component Mannich reactions with aromatic and aliphatic substrate scopes.Acknowledgements
This work was supported by the 111 Project (B14041), the grant from National Natural Science Foundation of China (21571121, 21271124, 21272186), the Fundamental Funds Research for the Central Universities (No. GK201501005), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R33) and Natural Science Basic Research Plan in Shaanxi Province of China (2012JM2006).Notes and references
- (a) E. A. Dustin, B. C. D. Raup, D. Holte and K. A. Scheidt, Nat. Chem., 2010, 2, 766 CrossRef PubMed; (b) S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209 CrossRef CAS PubMed; (c) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337 RSC; (d) R. Qiu, X. Xu, Y. Li, G. Zhang and L. Shao, Chem. Commun., 2009, 13, 1679 RSC.
- M. C. Lehman, J. B. Gary, P. D. Boyle, M. S. Sanford and E. A. Ison, ACS Catal., 2013, 3, 2304 CrossRef CAS.
- (a) R. Ding, K. Katebzadeh, L. Roman, K. E. Bergquist and U. M. Lindström, J. Org. Chem., 2005, 71, 352 CrossRef PubMed; (b) K. M. Engle, D. H. Wang and J. Q. Yu, J. Am. Chem. Soc., 2010, 132, 14137 CrossRef CAS PubMed; (c) D. J. Berrisford, C. Bolm and K. B. Sharpless, Angew. Chem., Int. Ed., 1995, 34, 1059 CrossRef CAS; (d) M. Hatano, Y. Furuya, T. Shimmura, K. Moriyama, S. Kamiya, T. Maki and K. Ishihara, Org. Lett., 2010, 13, 426 CrossRef PubMed; (e) B. Yu, W. Xu, H. Sun, B. Yu, G. Zhang, L. W. Xu, W. Zhang and Z. Gao, RSC Adv., 2015, 5, 8351 RSC.
- (a) P. A. Donets and N. Cramer, Angew. Chem., Int. Ed., 2015, 54, 633–637 CAS; (b) D. Zhang and G. Zi, Chem. Soc. Rev., 2015, 44, 1898–1921 RSC.
- (a) M. A. Mellmer, C. Sener, J. M. R. Gallo, J. S. Luterbacher, D. M. Alonso and J. A. Dumesic, Angew. Chem., Int. Ed., 2014, 53, 11872 CrossRef CAS PubMed; (b) M. E. Krafft, D. V. Vidhani, J. W. Cran and M. Manoharan, Chem. Commun., 2011, 47, 6707 RSC; (c) J. Kuwabara, W. Tsuchida and T. Kanbara, Polym. Chem., 2015, 6, 891 RSC; (d) S. Handa, F. Gallou and B. H. Lipshutz, Science, 2015, 349, 1087 CrossRef CAS PubMed.
- (a) J. Dudziak and J. D. Atwood, J. Coord. Chem., 2011, 64, 3575 CrossRef CAS; (b) M. N. Khan, I. L. Fatope, K. I. Isaac and M. O. Zubair, J. Chem. Soc., 1986, 2, 655 Search PubMed; (c) J. H. Clark, D. J. Macquarrie and J. Sherwood, Chem.–Eur. J., 2013, 19, 5174 CrossRef CAS PubMed.
- (a) A. Gansäuer, D. Franke, T. Lauterbach and M. Nieger, J. Am. Chem. Soc., 2005, 127, 11622 CrossRef PubMed; (b) G. Erker, G. Kehr and R. Froehlich, Coord. Chem. Rev., 2006, 250, 36 CrossRef CAS; (c) Titanium and Zirconium in Organic Synthesis, ed. I. Marek, Wiley-VCH: Weinheim, Germany, 2002 Search PubMed; (d) P. J. Chirik, Organometallics, 2010, 29, 1500 CrossRef CAS; (e) T. Saito, H. Nishiyama, H. Tanahashi, K. Kawakita, H. Tsurugi and K. Mashima, J. Am. Chem. Soc., 2014, 136, 5161 CrossRef CAS PubMed; (f) D. F. Chen, Z. Y. Han, X. L. Zhou and L. Z. Gong, Acc. Chem. Res., 2014, 47, 2365 CrossRef CAS PubMed; (g) M. E. Sloan, A. Staubitz, T. J. Clark, C. A. Russell, G. C. L. Jones and I. Manners, J. Am. Chem. Soc., 2010, 132, 3831 CrossRef CAS PubMed; (h) A. Zydor, V. G. Kessler and S. D. Elliott, Phys. Chem. Chem. Phys., 2012, 14, 7954 RSC; (i) A. M. Chapman and D. F. Wass, Dalton Trans., 2012, 41, 9067 RSC.
- A. Gansaeuer, I. Winkler, D. Worgull, D. Franke, T. Lauterbach, A. Okkel and M. Nieger, Organometallics, 2008, 27, 5699 CrossRef CAS.
- (a) A. A. Oluyadi, S. Ma and C. N. Muhoro, Organometallics, 2012, 32, 70 CrossRef; (b) D. Harmsen, G. Erker, R. Fröhlich and G. Kehr, Eur. J. Inorg. Chem., 2002, 12, 3156 CrossRef.
- T. Takeda, M. Ando, T. Sugita and A. Tsubouchi, Org. Lett., 2007, 9, 2875 CrossRef CAS PubMed.
- T. K. Hollis, N. P. Robinson and B. Bosnich, Organometallics, 1992, 11, 2745 CrossRef CAS.
- R. Qiu, X. Xu, L. Peng, Y. Zhao, N. Li and S. Yin, Chem.–Eur. J., 2012, 18, 6172 CrossRef CAS PubMed.
- Y. Wu, C. Chen, G. Jia, X. Zhu, H. Sun, G. Zhang, W. Zhang and Z. Gao, Chem.–Eur. J., 2014, 20, 8530 CrossRef CAS PubMed.
- (a) J. L. Li, C. Y. Zhang and L. X. Gao, J. Chem. Crystallogr., 2009 Search PubMed; (b) J. H. Toney and T. J. Marks, J. Am. Chem. Soc., 1985, 107, 947 CrossRef CAS; (c) R. Fandos, B. Gallego, A. Otero, A. Rodriguez, M. J. Ruiz, P. Terreros and C. Pastor, Organometallics, 2007, 26, 2896 CrossRef CAS.
- Q.-X. Guo, H. Liu, C. Guo, S.-W. Luo, Y. Gu and L.-Z. Gong, J. Am. Chem. Soc., 2007, 129, 3790–3791 CrossRef CAS PubMed.
- (a) A. F. A. Peacock, A. Habtemariam, R. Fernández, V. Walland, F. P. A. Fabbiani, S. Parsons, R. E. Aird, D. I. Jodrell and P. J. Sadler, J. Am. Chem. Soc., 2006, 128, 1739 CrossRef CAS PubMed; (b) D. Schröder, Acc. Chem. Res., 2012, 45, 1521 CrossRef PubMed.
- (a) C. J. Gibler, L. R. Chamberlain and R. J. Hoxmeier, US pat., 5242986A, 1993; (b) J. C. Huffman, K. G. Moloy, J. A. Marsella and K. G. Caulton, J. Am. Chem. Soc., 1980, 120, 3009–3014 CrossRef.
- (a) P. L. Yamamoto, Top. Organomet. Chem., 2011, 37, 161 CrossRef; (b) H. Y. Futatsugi, Angew. Chem., Int. Ed., 2005, 44, 1924 CrossRef PubMed.
- T. O. Y. Hayashi, T. Itoh, T. Urushima, H. Ishikawa and T. Uchimaru, Angew. Chem., 2008, 120, 9193 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27094d |
| This journal is © The Royal Society of Chemistry 2016 |