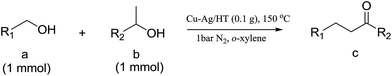
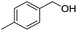
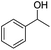
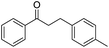
Cu–Ag/hydrotalcite catalysts for dehydrogenative cross-coupling of primary and secondary benzylic alcohols†
Jin Xuab,
Hongmei Yueab,
Sheng Liuab,
Hanfei Wangab,
Yuqun Duab,
Chunli Xu*ab,
Wensheng Dongab and
Chunling Liuab
aKey Laboratory of Applied Surface and Colloid Chemistry (Shaanxi Normal University), Ministry of Education, Xi'an 710119, PR China. E-mail: xuchunli@snnu.edu.cn; Tel: +86-29-81530779
bSchool of Chemistry and Chemical Engineering, Shaanxi Normal University, Chang'an West Street 620, Xi'an 710119, PR China
First published on 25th February 2016
Abstract
The development of new and inexpensive heterogeneous catalysts for direct C–C cross-coupling of primary and secondary alcohols is a challenging goal and has great importance in academic and industrial sectors. In this work Cu–Ag/hydrotalcite (Cu–Ag/HT) catalysts were prepared and tested for their impact on this cross-coupling. The effect of supports, including MgO, γ-Al2O3 and HT with different Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
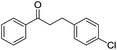
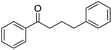
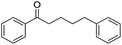
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratios, was investigated. It was found that the acidic or basic properties of the supports affected product selectivity. The roles of Cu and Ag sites in the cross-coupling were also investigated with the prepared Cu–Ag/HT catalyst demonstrating high activity and selectivity for the reaction. The yield-to-target product of β-phenylpropiophenone reached 99% after 1 h under optimum reaction conditions. The stability in air and reusability studies show that Cu–Ag/HT can be stored for 6 days and can be used five times without apparent deactivation, respectively.
Al molar ratios, was investigated. It was found that the acidic or basic properties of the supports affected product selectivity. The roles of Cu and Ag sites in the cross-coupling were also investigated with the prepared Cu–Ag/HT catalyst demonstrating high activity and selectivity for the reaction. The yield-to-target product of β-phenylpropiophenone reached 99% after 1 h under optimum reaction conditions. The stability in air and reusability studies show that Cu–Ag/HT can be stored for 6 days and can be used five times without apparent deactivation, respectively.
1. Introduction
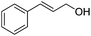
Acceptorless dehydrogenation reactions, in which hydrogen is liberated and new prospective bonds are generated by further reactions of the dehydrogenated products, are emerging as an atom-economical and environmentally benign synthetic method, circumventing the need for stoichiometric oxidants or the prefunctionalization of substrates.1,2 The self-coupling of an alcohol or the cross-coupling of two different alcohols constitutes a key transformation in reaction schemes targeting higher yields of alcohols, ketones, and esters.3 The homodimerization of primary and secondary alcohols, the so-called Guerbet reaction, has been known for more than a century.3,4 However, the reaction has recently been extended to the cross-coupling (selective β-alkylation) of secondary alcohols with primary alcohols (Scheme 1).5Several reports have demonstrated the β-alkylation of secondary alcohols with primary alcohols under the catalysis of Ru and Ir complexes.5–8 However, a number of limitations exist for these processes. These limitations include low catalytic turnover numbers, the requirement of a large amount of a strong base, and expensive catalysts, which are also difficult to synthesize and unable to be reused.9 These limitations reinforce the need for a ligand-free, reusable catalyst system for this type of transformation. To date, there are three related examples of heterogeneous catalysis.9–11 Ag/Al2O3 9 and Pd/C10 have been used as efficient catalysts for direct C–C cross-coupling of secondary and primary alcohols. However, a sacrificial hydrogen acceptor and/or a considerable amount of inorganic homogeneous base was required to achieve a high selectivity. Au–Pd/hydrotalcite (Au–Pd/HT) catalysts have been shown to avoid the need for a hydrogen acceptor and homogeneous base as well as exhibiting high catalytic performance.11 However, Au and Pd are precious metals with relatively scarce global reserves. The development of new and inexpensive heterogeneous catalysts for the reaction is therefore a challenging goal and has great importance in academic and industrial sectors.12
The reaction proceeds by dehydrogenation of the alcohol to an intermediate carbonyl compound. This is followed by an aldol condensation with the ketone to yield an α,β-unsaturated carbonyl derivative, which is finally reduced by the metal hydride to give the corresponding carbonyl compound (Scheme 2).9 A key concept in the catalyst design in some of the systems is the multifunctionality of metal-loaded acidic and/or basic metal oxides. Here, the acidic and/or basic sites selectively catalyze the aldol condensation reactions while the metal sites catalyze the transfer dehydrogenation of alcohol and transfer hydrogenation of the condensation products as intermediates.5,13 Catalysts based on Cu have attracted much attention in heterogeneous catalysis because of the metal's relative cost advantage.14–16 Such catalysts have been reported to provide high activity in the dehydrogenation of alcohols and hydrogen transfer.17,18 The catalytic performance of Cu was reportedly affected by the coexistence of Ag nanoparticles.19,20 HT showed high activity for the aldol condensation.11,21 Therefore, Cu–Ag/HT may offer the potential for catalyzing the reaction, the investigation of which is the focus of this work.
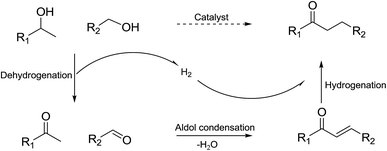 | ||
| Scheme 2 Mechanism for the cross-coupling of primary and secondary alcohols to produce higher ketones. | ||
2. Experimental section
2.1 Catalyst preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ molar ratios with a total concentration of 1.5 M was mixed slowly by continuous stirring with an alkaline solution of Na2CO3–NaOH. The molar ratio of Na2CO3 to Al3+ was 2
Al3+ molar ratios with a total concentration of 1.5 M was mixed slowly by continuous stirring with an alkaline solution of Na2CO3–NaOH. The molar ratio of Na2CO3 to Al3+ was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pH of the mixture was maintained between a value of 9 and 10 by adjusting the flow rate of the alkaline solution. The temperature was maintained at 25 °C. Following this addition, which resulted in the formation of heavy slurry, the mixture was aged at 60 °C for 18 h by stirring to selectively facilitate the growth of the precipitated HT phase. The slurry was then cooled to 25 °C, filtered, and washed with water until the pH value of the filtrate was approximately 7. The precipitate was dried at 110 °C for 12 h. Depending on the initial conditions, the resulting material was HT with a Mg2+
1. The pH of the mixture was maintained between a value of 9 and 10 by adjusting the flow rate of the alkaline solution. The temperature was maintained at 25 °C. Following this addition, which resulted in the formation of heavy slurry, the mixture was aged at 60 °C for 18 h by stirring to selectively facilitate the growth of the precipitated HT phase. The slurry was then cooled to 25 °C, filtered, and washed with water until the pH value of the filtrate was approximately 7. The precipitate was dried at 110 °C for 12 h. Depending on the initial conditions, the resulting material was HT with a Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ molar ratio of 1
Al3+ molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, or 5
1, or 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Hereafter, unless noted otherwise, HT describes a 2
1. Hereafter, unless noted otherwise, HT describes a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Mg2+
1 molar ratio of Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+.
Al3+.A mesoporous MgO support for the Cu–Ag catalyst was prepared by calcining commercially available Mg(OH)2 (99%, Guangdong Shantou Hongwei Chemical Plant, P. R. China) at 500 °C for 3 h in static air (muffle oven, heating rate: 10 °C min−1). A γ-Al2O3 support was prepared by calcining a commercial sample of γ-Al2O3 (Alfa Aesar) under the same conditions.
2.2 Characterization of catalyst
The surface area and pore characteristics of the catalysts were determined using a Micromeritics ASAP 2020 instrument (USA). The sample was degassed at 110 °C for 6 h in N2 prior to carrying out surface area measurements. Nitrogen adsorption and desorption isotherms were measured at −196 °C. The specific surface areas of the catalysts were determined by applying the Brunauer–Emmett–Teller (BET) method to the nitrogen adsorption data obtained in the relative pressure range of 0.06 to 0.30. The total pore volume of the samples was estimated from the amount of nitrogen adsorbed at a relative pressure of 0.995. Pore volume and pore size distributions were obtained from analysis of the desorption branches of the nitrogen isotherms using the Barrett–Joyner–Halenda method.X-ray diffraction (XRD) patterns were recorded using a D/Max-3C X-ray powder diffractometer (Rigaku Co, Japan), with a Cu–K source fitted with an Inel CPS 120 hemispherical detector.
Scanning transmission electron microscopy (STEM) micrographs were obtained using a FEI Tecnai G2 F20 instrument (USA).
Elemental analysis of HT was carried out by an FEI Quanta 200 (USA) scanning electron microscope equipped with energy-dispersive X-ray spectroscopy.
The loading of Cu–Ag was determined by inductively coupled plasma mass spectrometry (Bruker, M90; Germany).
X-ray photoelectron spectroscopy (XPS) was used to characterize the valence of catalysts. XPS data was obtained in an Axis Ultra spectrometer (Kratos Analytical Ltd., Japan) using AlKα (hυ = 1486.6 eV) X-ray radiation at an energy of 150 W.
NH3-temperature-programmed desorption (TPD) and CO2-TPD were performed on a Micromeritics AutoChem 2920 II instrument. Typically, the sample loaded in a quartz reactor was pretreated with high-purity He at 350 °C for 1 h. After cooling the sample to 100 °C, CO2 adsorption was performed by switching the He flow to a CO2–Ar (5 vol% CO2) gas mixture and then maintaining the sample at 100 °C for 3 h. The gas-phase (and/or weakly adsorbed) CO2, was purged by high-purity He at the same temperature. CO2-TPD was then performed in the He flow by raising the temperature to 800 °C at a rate of 10 °C min−1. The desorbed CO2 molecules were detected by using a MKS Cirrus 2 mass spectrometer. NH3-TPD was performed by using a similar procedure.
2.3 Reaction procedure
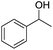
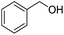
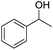
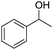
The cross-coupling of primary and secondary alcohols was carried out using a 50 mL, three-necked, round-bottomed flask with a water-cooled condenser. Typically, the reactor was charged with 0.1 g of catalyst, 1.0 mmol of benzyl alcohol, 1.0 mmol of 1-phenylethanol, and 3 mL of o-xylene. This mixture was then heated at 150 °C for 1 h under N2 at 1 atm. After cooling to room temperature, the resulting mixture was diluted to a constant volume with toluene in a 15 mL volumetric flask. The reaction products were quantitatively analyzed by a Shimadzu GC-2014 (Rtx®-5, 30 m × 0.32 mm × 0.25 μm) gas chromatograph equipped with a flame ionization detector. N-Dodecane (1 mmol) was used as the internal standard for analysis. The products were identified by 1H NMR as well as GC-MS.3. Results and discussion
3.1 Catalyst characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio (from 1
Al ratio (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The surface area of Cu–Ag/γ-Al2O3 (Table 1, entry 1) was similar to that of Cu–Ag/HT. Cu–Ag/MgO had the lowest surface area of 125 m2 g−1. The isotherms of the six samples exhibited the characteristic type IV shape, which, together with the pore size distribution results, suggested that the samples were mesoporous materials (Fig. 1).
1). The surface area of Cu–Ag/γ-Al2O3 (Table 1, entry 1) was similar to that of Cu–Ag/HT. Cu–Ag/MgO had the lowest surface area of 125 m2 g−1. The isotherms of the six samples exhibited the characteristic type IV shape, which, together with the pore size distribution results, suggested that the samples were mesoporous materials (Fig. 1).
| Entry | Samples | Metal ratio (Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al)a Al)a |
Surface areab (m2 g−1) | Pore volumec (cm3 g−1) | Pore diameter (Å) |
|---|---|---|---|---|---|
| Prepared/determined | |||||
| a Determined by energy-dispersive X-ray spectroscopy.b Calculated by the BET method.c Calculated by the Barrett–Joyner–Halenda method from the desorption isotherm. | |||||
| 1 | Cu–Ag/γ-Al2O3 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/0 1/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
200 | 0.58 | 78 |
| 2 | Cu–Ag/HT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1.25 1/1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
229 | 1.12 | 127 |
| 3 | Cu–Ag/HT | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2.08 1/2.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
198 | 0.60 | 102 |
| 4 | Cu–Ag/HT | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2.96 1/2.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
209 | 0.66 | 96 |
| 5 | Cu–Ag/HT | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/5.63 1/5.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
202 | 0.49 | 73 |
| 6 | Cu–Ag/MgO | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/1 0/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
125 | 0.44 | 105 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Mg
1 molar ratio of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al showed major diffraction peaks for HT and minor diffraction peaks for Al2O3 (Fig. 2a). For HT supports with molar ratios of 2
Al showed major diffraction peaks for HT and minor diffraction peaks for Al2O3 (Fig. 2a). For HT supports with molar ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 5
1, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the results were the same as those for HT, with characteristic diffraction peaks at 11.4, 23.0, and 34.9° (Fig. 2b–d).22 Cu–Ag/HT results showed the diffraction peaks characteristic of MgO, which were observed irrespective of the Mg
1, the results were the same as those for HT, with characteristic diffraction peaks at 11.4, 23.0, and 34.9° (Fig. 2b–d).22 Cu–Ag/HT results showed the diffraction peaks characteristic of MgO, which were observed irrespective of the Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio and distinct from the corresponding peaks from their HT supports (Fig. 2e). In the XRD spectra of Cu–Ag/HT, a diffuse diffraction diagram of MgO only was observed, and Al2O3 formed a separate amorphous phase without diffraction pattern in XRD.23–25 This indicated that the HT supports were transformed into mixed oxide in the process of preparing Cu–Ag/HT catalysts. Results for γ-Al2O3 and Cu–Ag/γ-Al2O3 were similar, showing the typical features of γ-Al2O3 (Fig. 3).22 This indicated that the crystalline structure of the γ-Al2O3 supports did not change after supporting the Cu–Ag phase. Results for MgO and Cu–Ag/MgO were also similar to each other. No reflections corresponding to Cu or Ag were observed for catalysts loaded with 1 wt% of catalyst because of the low loadings (Fig. 2 and 3). XRD patterns of 10 wt% Cu–Ag/HT were also determined (Fig. 4) and show reflections corresponding to Cu because of the increased catalyst loading.
Al molar ratio and distinct from the corresponding peaks from their HT supports (Fig. 2e). In the XRD spectra of Cu–Ag/HT, a diffuse diffraction diagram of MgO only was observed, and Al2O3 formed a separate amorphous phase without diffraction pattern in XRD.23–25 This indicated that the HT supports were transformed into mixed oxide in the process of preparing Cu–Ag/HT catalysts. Results for γ-Al2O3 and Cu–Ag/γ-Al2O3 were similar, showing the typical features of γ-Al2O3 (Fig. 3).22 This indicated that the crystalline structure of the γ-Al2O3 supports did not change after supporting the Cu–Ag phase. Results for MgO and Cu–Ag/MgO were also similar to each other. No reflections corresponding to Cu or Ag were observed for catalysts loaded with 1 wt% of catalyst because of the low loadings (Fig. 2 and 3). XRD patterns of 10 wt% Cu–Ag/HT were also determined (Fig. 4) and show reflections corresponding to Cu because of the increased catalyst loading.
To study the resistance to oxidation of Cu–Ag bimetallic nanoparticles, XPS spectra of Cu–Ag/HT were taken after samples had been stored for 2 or 3 months (Fig. 6). Spectra of Cu 2p for Cu–Ag/HT samples that had been stored showed the characteristics of metallic Cu and were similar to the results from the fresh catalysts. In contrast, the XPS spectra of Cu 2p for Cu/HT samples that had been stored exhibited peaks associated with Cu2+ alongside the characteristic peaks of metallic Cu.20,26 This indicated that part of the Cu nanoparticles on Cu/HT had been oxidized to Cu2+ by air. This also suggested that the Cu atoms of the Cu–Ag bimetallic nanoparticles were less oxidized than those in the pure Cu nanoparticles. The enhanced resistance to oxidation of Cu–Ag bimetallic nanoparticles was ascribed to the addition of Ag.20 The structure and resistance to oxidation of Cu–Ag bimetallic nanoparticles have been investigated in the literature.20,27–30 The results from the literature about Cu–Ag nanoparticles indicated that Cu nanoparticles are easily oxidized, and the addition of Ag to Cu enhanced the resistance of Cu to oxidation. The enhanced resistance was ascribed to the smaller number of surface-exposed Cu atoms compared to the entire Cu atoms in this Ag–Cu bimetallic nanoparticles and electronic interaction between Ag and Cu atoms in the Ag–Cu bimetallic nanoparticles.
 | ||
| Fig. 7 STEM images and SAED patterns of Cu–Ag/HT catalysts calcined and reduced at (a) 300 °C and (b) 600 °C. Inset: HR-TEM images of the selected area. | ||
The SAED patterns indicated that the Cu–Ag nanoparticle was polycrystalline. Each ring of the SAED image is a reflection of planes with different interplanar spacings.20 Using the length of scale (L) and the radius of the diffraction ring (R) in the equations L/5 = R/N and X = 1/N (where N is the reciprocal spacing and X is the spacing), X values of 2.47, 1.79, and 1.33 Å were obtained. The spacing values of nanoparticles in the samples were consistent with those of lattice planes (111), (200) and (220) of Cu nanoparticles. Combined with the XPS and XRD analysis, it can be concluded that both Cu and Ag nanoparticles were in a metallic state.
3.2 Effect of supports
Analysis of γ-Al2O3, HT with different Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ molar ratios, and MgO supports (Fig. 9) revealed that the supports affected both the activity and selectivity of Cu–Ag catalysts. When γ-Al2O3 was used as support, the conversion of benzyl alcohol was 75% (Fig. 9a). In the case of HT supports, the conversion of benzyl alcohol varied with the Mg2+
Al3+ molar ratios, and MgO supports (Fig. 9) revealed that the supports affected both the activity and selectivity of Cu–Ag catalysts. When γ-Al2O3 was used as support, the conversion of benzyl alcohol was 75% (Fig. 9a). In the case of HT supports, the conversion of benzyl alcohol varied with the Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ molar ratio. At a molar ratio of 1
Al3+ molar ratio. At a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 the conversion was 50%, but this increased to a maximum of 88% when the molar ratio was 2
1 the conversion was 50%, but this increased to a maximum of 88% when the molar ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Above 2
1. Above 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion decreased as Mg2+
1, the conversion decreased as Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ was increased. When pure MgO was used as the support the conversion of benzyl alcohol was 64%.
Al3+ was increased. When pure MgO was used as the support the conversion of benzyl alcohol was 64%.
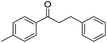
The reaction produced two types of products (Scheme 3). Ether was formed from the direct etherification of primary and secondary benzylic alcohols. Alternatively, β-phenylpropiophenone was formed by dehydrogenative cross-coupling of primary and secondary benzylic alcohols. When γ-Al2O3 was used as the support, ethers were produced (Fig. 9b). In contrast, when HT or MgO were the support only β-phenylpropiophenone was found. Furthermore, the yield of β-phenylpropiophenone varied with ratio of Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+; at 1
Al3+; at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 the yield was 21%, but this increased to the maximum of 76% at 2
1 the yield was 21%, but this increased to the maximum of 76% at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Above 2
1. Above 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield decreased as Mg2+
1, the yield decreased as Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ was increased. When pure MgO was used as the support, the yield of β-phenylpropiophenone decreased to 35%. Notably, α,β-unsaturated carbonyl derivatives, for example, the intermediate 1,3-diphenyl-acrylketone, were not observed in the presence of Cu–Ag/HT or Cu–Ag/MgO.
Al3+ was increased. When pure MgO was used as the support, the yield of β-phenylpropiophenone decreased to 35%. Notably, α,β-unsaturated carbonyl derivatives, for example, the intermediate 1,3-diphenyl-acrylketone, were not observed in the presence of Cu–Ag/HT or Cu–Ag/MgO.
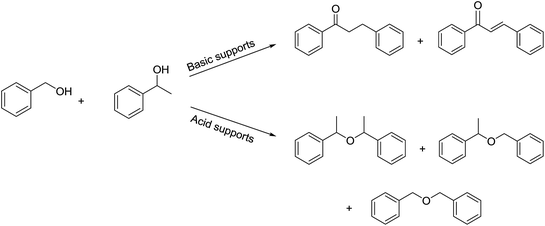 | ||
| Scheme 3 Dehydrogenative cross-coupling or direct etherification of primary and secondary benzylic alcohols. | ||
The effect of the supports on the reaction selectivity was ascribed to whether the supports were acidic or basic. Acidic sites promoted the etherification of primary and secondary benzylic alcohols.33 Conversely, strongly basic sites catalyzed the aldol condensation step in the dehydrogenative cross-coupling of primary and secondary benzylic alcohols.5,13 γ-Al2O3 includes weakly acidic sites,34 while HT and MgO have strong basic sites.35 This explains why the main product was ether for Cu–Ag/γ-Al2O3, and β-phenylpropiophenone for Cu–Ag/HT and Cu–Ag/MgO.
The activity of pure, undoped γ-Al2O3 was also tested for the reaction between primary and secondary benzylic alcohols (Table 2). Here, as with Cu–Ag/γ-Al2O3, the products were also ethers, indicating that either Cu or Ag failed to function with the γ-Al2O3 support.
| Entry | Reaction time | Conversion of benzyl alcohol (%) | Yields (%) | ||
|---|---|---|---|---|---|
| Dibenzyl ether | 1-Methyl benzyl ether | 1,1′-Diphenyl ether | |||
| a Reaction conditions: benzyl alcohol (1.0 mmol), 1-phenylethanol (1.0 mmol), catalyst (0.1 g), o-xylene (3 mL), 150 °C, 1 bar N2. | |||||
| 1 | 45 min | 81 | 0.2 | 49 | 2.9 |
| 2 | 1 h | 87 | 0.4 | 58 | 3.6 |
| 3 | 1.5 h | 88 | 0.3 | 69 | 4.0 |
| 4 | 2 h | 99 | 0.5 | 95 | 2.6 |
3.3 Effect of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Ag mass ratio
Ag mass ratio
Fig. 10 shows that the conversion of benzyl alcohol and yield of β-phenylpropiophenone were lowest for the reaction using pure Ag, the latter being almost zero. Increasing the Cu content increased both the conversion and the yield with respective maximums of 88 and 76% observed after 0.5 h when the content of Cu was 95%. Using pure Cu caused the conversion and yield after 0.5 h to fall to 68% and 53%, respectively. These results suggested that the main active site was Cu, but that its activity was promoted by the presence of Ag. As shown in Fig. 6, the addition of Ag enhanced the resistance to oxidation of Cu. The role of Ag in the activity of Cu–Ag may be related to the increase in the resistance to oxidation of Cu. Besides, the presence of Ag may change the electronic and morphological structure of Cu, which affected the activity of Cu. The role of Ag will be studied in the future work.
3.4 Catalytic performance
The effect of calcination temperature and reaction conditions was studied (Fig. S1–S3, and Tables S1 and S2†). The results indicated that the optimum reaction conditions involved using 0.1 g of catalyst in a reaction at a temperature of 150 °C that lasted 1 h. Under these conditions both the conversion of benzyl alcohol and the yield of β-phenylpropiophenone approached 99%.Liu et al.11 reported that Au–Pd/HT catalysts showed high catalytic performance for the reaction studied here. Indeed, they reported that both conversion and yield neared 97% after 5 h for a reaction involving primary alcohol (1 mmol), secondary alcohol (1 mmol), p-xylene (3 mL), and the Au–Pd/HT catalyst (0.38 g) in a N2 atmosphere at 120 °C and 1 bar. Results for similar reaction conditions using Cu–Ag/HT catalyst showed that both the conversion of benzyl alcohol and the yield of β-phenylpropiophenone were similar to those obtained with Au–Pd/HT. Therefore, and given that Cu and Ag are more widely available and less expensive than Au and Pd, we suggest that Cu–Ag/HT is a better choice of catalyst for the dehydrogenation cross-coupling reaction of primary and secondary alcohols.
3.5 Coupling with various alcohols
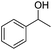
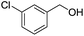
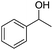
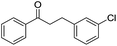
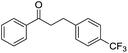
With suitable reaction conditions established, the scope of substrates was then investigated (Table 3). Very high yields (90–100%) of the corresponding cross-coupled products were obtained from 1-phenylethanol and various substituted benzyl alcohols, including both electron-rich and electron-deficient examples (Table 3, entries 1–5). Notably, electron-donating substituents on the primary alcohol appeared to play a significant role, shortening the reaction time required to achieve completion (entries 1 and 2). Conversely, m-substituted alcohols appeared to exhibit lower reaction rates and yields than p-substituted alcohols, likely because of steric effects (entries 3 and 4). Similarly, excellent yields (97–99%) of the corresponding cross-coupled products were also obtained when using various substituted 1-phenyl-ethanol and benzyl alcohols, again including both electron-rich and electron-deficient examples (entries 6 and 7).The results also suggest that aliphatic primary alcohols are not suitable coupling partners. For example, when 2-phenylethanol was employed as the substrate, complete conversion and complete yield were obtained after 8 h (entry 8). However, when cinnamyl primary alcohol was used as the substrate, the corresponding yield decreased to 58% after the same reaction period (entry 9). Unfortunately, after 8 h none of the desired cross-coupled product was detected if aliphatic primary alcohols without benzene substituents, such as allyl alcohol or propyl alcohol, were employed as substrates (entries 10 and 11). Instead these cases produced 1,3-diphenyl-1-butanone, which resulted from the self-coupling of 1-phenylethanol.
For the reactions of entries 10 and 11 (Table 3), allyl alcohol or propyl alcohol kept unchanged when 1-phenylethanol occurred the dehydrogenative self-coupling. In the absences of allyl alcohol or propyl alcohol, 1-phenylethanol still occurred the dehydrogenative self-coupling in similar yield. It was found that the cross-coupling and self-coupling competed with each other in the primary and secondary mixed alcohol system. As shown in Scheme 2, the first step of the reaction proceeds by dehydrogenation of the alcohol to an intermediate carbonyl compound. The dehydrogenation of aliphatic primary alcohols may go slowly in the presence of Cu–Ag/HT catalysts,36,37 whereas the rate of dehydrogenation of 1-phenylethanol was high. The low rate in dehydrogenation of aliphatic primary alcohols decreased the rate of cross-coupling between aliphatic primary alcohol and 1-phenylethanol, but did not affect the dehydrogenative self-coupling of 1-phenylethanol. This explained the reactions of entries 10 and 11. However, for reactions of entries 1–9, the detected products were mainly from the cross-coupling of primary and secondary alcohol, but not the self-coupling of alcohol. Furthermore, the yield to self-coupling of alcohol was very low and negligible. The high yield to cross-coupling of primary and secondary alcohol in entries 1–9 was mainly ascribed to the property of the involved primary alcohol, which was various substituted benzyl alcohols. The various substituted benzyl alcohols was easily dehydrogenated to form the corresponding aldehyde,36,37 accordingly, easily cross-coupled with the secondary alcohol.
3.6 Stability in air and reusability of catalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but this decreased to almost 1.2
1, but this decreased to almost 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after nine uses. The decrease in loading amount of Cu and Mg2+
1 after nine uses. The decrease in loading amount of Cu and Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ molar ratio showed that both Cu and Mg2+ could leach into the reaction system. Further work will therefore focus on the prevention of the observed leaching of Cu and Mg2+ into the reaction system.
Al3+ molar ratio showed that both Cu and Mg2+ could leach into the reaction system. Further work will therefore focus on the prevention of the observed leaching of Cu and Mg2+ into the reaction system.
| Entry | Used times | Conversion of benzyl alcohol (%) | Yield to β-phenylpropiophenone (%) |
|---|---|---|---|
| a The spent catalyst was washed with o-xylene three times, and then applied to the further run without any other treatment. Reaction conditions: primary alcohol (1.0 mmol), secondary alcohol (1.0 mmol), o-xylene (3 mL), Cu–Ag/HT (0.1 g), 150 °C, 1 bar N2, 1 h. | |||
| 1 | 1st | 98 | 98 |
| 2 | 2nd | 98 | 98 |
| 3 | 3rd | 98 | 98 |
| 4 | 4th | 98 | 95 |
| 5 | 5th | 93 | 90 |
| 6 | 6th | 85 | 80 |
| 7 | 7th | 78 | 57 |
| 8 | 8th | 55 | 24 |
| 9 | 9th | 56 | 0.8 |
4. Conclusions
Acidic/basic properties of catalyst supports affected the activity and selectivity of Cu–Ag/HT in the reactions studied here. Acidic supports promoted the etherification of primary and secondary benzylic alcohols, whereas basic supports advanced the dehydrogenative cross-coupling of primary and secondary benzylic alcohols. Cu–Ag/HT with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Mg to Al showed the highest activity and demonstrated it to be an active catalyst for the C–C cross-coupling of secondary and primary alcohols. The active sites of Cu–Ag/HT were Cu, with Ag involved in promoting the activity of Cu sites as well as hindering their oxidation. The yield of β-phenylpropiophenone reached 99% after 1 h under optimum reaction conditions. The catalyst also displayed reusability, with no loss of activity after five cycles, and resistance to oxidation, being able to be stored for 6 days without deactivation. However, the Cu–Ag/HT catalyst was completely deactivated after more than nine uses because of leaching of Cu and Mg2+ into the reaction system.
1 molar ratio of Mg to Al showed the highest activity and demonstrated it to be an active catalyst for the C–C cross-coupling of secondary and primary alcohols. The active sites of Cu–Ag/HT were Cu, with Ag involved in promoting the activity of Cu sites as well as hindering their oxidation. The yield of β-phenylpropiophenone reached 99% after 1 h under optimum reaction conditions. The catalyst also displayed reusability, with no loss of activity after five cycles, and resistance to oxidation, being able to be stored for 6 days without deactivation. However, the Cu–Ag/HT catalyst was completely deactivated after more than nine uses because of leaching of Cu and Mg2+ into the reaction system.
Acknowledgements
This work was supported by projects funded by the Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2013JM2003) and by the Fundamental Research Funds for the Central Universities (Program No. GK201505002).References
- C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712 CrossRef PubMed.
- X. Liu, L. He, Y. Liu and Y. Cao, Acc. Chem. Res., 2014, 47, 793 CrossRef CAS PubMed.
- I. S. Makarov and R. Madsen, J. Org. Chem., 2013, 78, 6593 CrossRef CAS PubMed.
- J. T. Kozlowski and R. J. Davis, ACS Catal., 2013, 3, 1588 CrossRef CAS.
- K. Shimizu, Catal. Sci. Technol., 2015, 5, 1412 CAS.
- S. Musa, L. Ackermann and D. Gelman, Adv. Synth. Catal., 2013, 355, 3077 CrossRef CAS.
- D. Gnanamgari, C. H. Leung, N. D. Schley, S. T. Hilton and R. H. Crabtree, Org. Biomol. Chem., 2008, 6, 4442 CAS.
- D. Wang, K. Zhao, X. Yu, H. Miao and Y. Ding, RSC Adv., 2014, 4, 42924 RSC.
- K. Shimizu, R. Sato and A. Satsuma, Angew. Chem., 2009, 121, 4042 CrossRef.
- C. S. Cho, W. X. Ren and S. C. Shim, Bull. Korean Chem. Soc., 2005, 26, 1611 CrossRef CAS.
- X. Liu, R. Ding, L. He, Y. Liu, Y. Cao, H. He and K. Fan, ChemSusChem, 2013, 6, 604 CrossRef CAS PubMed.
- L. Zhang, A. Wang, W. Wang, Y. Huang, X. Liu, S. Miao, J. Liu and T. Zhang, ACS Catal., 2015, 5, 6563 CrossRef CAS.
- J. D. Lewis, S. V. Vyver and Y. Román-Leshkov, Angew. Chem., Int. Ed., 2015, 54, 9835 CrossRef CAS PubMed.
- P. Fakhri, B. Jaleh and M. Nasrollahzadeh, J. Mol. Catal. A: Chem., 2014, 383–384, 17 CrossRef CAS.
- G. Corro, S. Cebada, U. Pal, J. L. G. Fierro and J. Alvarado, Appl. Catal., B, 2015, 165, 555 CrossRef CAS.
- Y. Zhu, X. Kong, S. Zhu, F. Dong, H. Zheng, Y. Zhu and Y. Li, Appl. Catal., B, 2015, 166–167, 551 CrossRef CAS.
- D. Damodara, R. Arundhathi and P. R. Likhar, Adv. Synth. Catal., 2014, 356, 189 CrossRef CAS.
- F. Santoro, R. Psaro, N. Ravasio and F. Zaccheria, RSC Adv., 2014, 4, 2596 RSC.
- K. Shimizu, K. Shimura, M. Nishimura and A. Satsuma, RSC Adv., 2011, 1, 1310 RSC.
- N. R. Kim, K. Shin, I. Jung, M. Shim and H. M. Lee, J. Phys. Chem. C, 2014, 118, 26324 CAS.
- Z. Wang, P. Fongarland, G. Lu and N. Essayem, J. Catal., 2014, 318, 108 CrossRef CAS.
- C. Xu, J. Sun, B. Zhao and Q. Liu, Appl. Catal., B, 2010, 99, 111 CrossRef CAS.
- J. M. L. Nieto, A. Dejoz and M. I. Vazquez, Appl. Catal., A, 1995, 132, 41 CrossRef.
- Y. Z. Chen, C. M. Hwang and C. W. Liaw, Appl. Catal., A, 1998, 169, 207 CrossRef CAS.
- A. Villa, A. Gaiassi, I. Rossetti, C. L. Bianchi, K. Benthem, G. M. Veith and L. Prati, J. Catal., 2010, 275, 108 CrossRef CAS.
- J. P. Espinós, J. Morales, A. Barranco, A. Caballero, J. P. Holgado and A. R. González-Elipe, J. Phys. Chem. B, 2002, 106, 6921 CrossRef.
- Z. Chen, D. Mochizuki, M. M. Maitani and Y. Wada, Nanotechnology, 2013, 24, 265602 CrossRef PubMed.
- J. Czaplinska, I. Sobczak and M. Ziolek, J. Phys. Chem. C, 2014, 118, 12796 CAS.
- K. D. Malviya and K. Chattopadhyay, J. Phys. Chem. C, 2014, 118, 13228 CAS.
- H. Peng, W. Qi, S. Li and W. Ji, J. Phys. Chem. C, 2015, 119, 2186 CAS.
- A. Cao, R. Lu and G. Veser, Phys. Chem. Chem. Phys., 2010, 12, 13499 RSC.
- Y. Wang, J. He, C. Liu, W. Chong and H. Chen, Angew. Chem., Int. Ed., 2015, 54, 2022 CrossRef CAS PubMed.
- E. R. Sacia, M. Balakrishnan and A. T. Bell, J. Catal., 2014, 313, 70 CrossRef CAS.
- J. Luo, J. Yu, R. J. Gorte, E. Mahmoud, D. G. Vlachos and M. A. Smith, Catal. Sci. Technol., 2014, 4, 3074 CAS.
- C. Xu, Y. Du, C. Li, J. Yang and G. Yang, Appl. Catal., B, 2015, 164, 334 CrossRef CAS.
- T. Mitsudome, Y. Mikami, K. Ebata, T. Mizugaki, K. Jitsukawa and K. Kaneda, Chem. Commun., 2008, 4804 RSC.
- T. Mitsudome, Y. Mikami, H. Funai, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., 2008, 120, 144 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22542f |
| This journal is © The Royal Society of Chemistry 2016 |