Construction and functional effects of a compatible nano-TiO2 interface between wood fiber and polypropylene via adopting chemical polyethyleneimine–(3-aminopropyl) triethoxysilane assembly on the fiber surface
Xianzhu Ye,
Hua Wang*,
Kang Zheng,
Zhaofeng Wu,
Haifeng Zhou and
Xingyou Tian*
Institute of Applied Technology, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, People’s Republic of China. E-mail: xytian@issp.ac.cn; wanghua@issp.ac.cn; Fax: +86 5515591434; Tel: +86 551 5591418
First published on 18th January 2016
Abstract
A simple chemical assembly to construct a compatible nano-TiO2-coating on a wood surface has been reported using polyethyleneimine and (3-aminopropyl) triethoxysilane for recycled wood–polypropylene composites (WPCs). Crystal features, ZETA potential, and FTIR analysis indicated that the assembly was stable and sufficient, which could be verified further using SEM imaging. Via a three-dimensional hydrophobic network in the composite, water absorbance decreased from 2.89 wt% to 0.72 wt%. Ultraviolet and near-infrared resistance were found to be increased for the dispersive nano-network in the composite matrix. In particular, when the assembling nano-interface was 2.0 wt% for the composite, as well as the tensile strength, the elongation at break was enhanced by about 85%. Tg was also enhanced for strong interfacial adhesion and sufficient load transfer. Also, due to the shielding of the nano-network and the coupling aspect of the assembly, the decomposition temperature (Td) of wood and polypropylene were enhanced by 79 °C and 27 °C, respectively.
1. Introduction
Recently, for resource recycling and environment protection, the interest in recycled Wood-Plastic-Composites (WPCs) reinforced by structure treatments has increased considerably due to their potential properties, including mechanical properties, thermal stability, and hydrophobicity, facilitating the use of the coupling effect and nano-technology in these materials.1,2 For instance, maleic anhydride coupled the wood fibers and plastic efficiently, together with three surface treatments, and the final mechanical properties were apparently enhanced.3 Furthermore, some reports showed micro-sized glass and carbon fibers and nano-sized montmorillonite could be used to reinforce a polypropylene/wood fiber composite if dispersed well.4–7 Due to weak activity and bad dispersion, conventional coupling routes in the compounding process do not have sufficient interactions if the coupling agents are added directly. Additionally, significant challenges remain for the development and the applications of high performance nano/WPCs including the need for (i) uniform dispersion of nano-particles in the WPC matrix and (ii) strong interfacial interactions for effective load transfer from the WPC matrix to the nano-structure. Thus, due to the low efficiency of the physical route, which cannot satisfy the requirements for the functions and commercial cost completely, chemical nano-assembly for a stable nano-interface may be a better strategy for nano-WPC production.Some new designs based on wood structure have already come into view. In situ polymerization and ion absorption on wood surfaces have been introduced into WPCs to construct a three-dimensional coating network.6,8–15 Particularly to assemble nano-coatings in ultrasonic environments, self-assembly via ion absorption can be efficient due to its sufficient charge interactions, simple synthetic process and efficient chemical stability. E.g. M. Agarwal stated that conductive paper from wood fibers coated with a nano-composite of carbon nanotubes and conductive polymers could be constructed well, and the conductive property could be controlled efficiently.16 Similarly, chemical assembly can take advantage of the hydroxyl groups of nano-particles and wood fibers to construct a stable reaction and a dispersed nano-interface in WPCs, which is unique and meaningful due to its chemical features when compared with conventional nano-modifications.
Nano-titanium dioxide (TiO2), endowed with excellent structural features and surface activity, has been widely used in mechanical reinforcements and antimicrobial materials.6 Additionally, nano-TiO2 is low cost and non-toxic, and has high chemical stability, and the ability to absorb or scatter UV irradiation. This material, due to its active groups on the surface and dispersed features, has a potential use in this work as a filler for WPCs. By making use of the hydroxyl groups on nano-surfaces, branched polyethyleneimine (PEI) can react with TiO2-particles acutely due to its high electron density and activity in particular, thus making nano-TiO2 cationic and dispersive, which can also solve aggregation to a certain extent due to charge repulsion. In addition, (3-aminopropyl) triethoxysilane (KH550) is used in various polymer composites due to its cross-linking property effect on the toughness, impact strength and creep properties, and sufficient activity allows it to graft with wood fibers with good efficiency, which has been demonstrated efficiently when used as a silane coupling.3,17,18 Finally, in this work, by making use of interactions between amidogen groups and silicon-hydroxyl groups, a chemical assembly to build a compatible nano-TiO2 interface between PEI-TiO2 and KH550-wood in WPCs was studied.
Except for a dispersed nano-network, the PEI and KH550 used in this work also provide the wood fibers with good compatibility with the polymer matrix due to their efficient molecular liquidity and grafting activity, which are meaningful to hydrophobicity, mechanical reinforcement, and thermal shielding. The assembly process in this work adopted ultrasonic technology to keep combinations stable. Via different assembly ratios, the assembling effect was mainly calculated using the ZETA potential, nano-particle size, grafted structure, crystal features and the assembled morphology. Particular emphasis in this paper was also placed on examining the internal mechanism of compatible nano-networks in WPCs as well as the relative functions of the wood–polypropylene composite, including dimensional stability, absorption and reflection in the ultraviolet-visible-near infrared region, mechanical reinforcements, and thermal shielding.
2. Materials and methods
2.1 Materials
Polypropylene (PP) particles were obtained from Dushanzi petrochemical company of China petroleum (Q/XJ 1100-1998 T30S, diameter: 3 mm). Wood powders were obtained from the Ltd of Shanghai Jia Feng (recycled poplar powders, monomodal width: 5–20 μm, monomodal length: 30–50 μm). Ethylene imine polymer (PEI, branched, the relative molecular mass is about 7000), (3-aminopropyl) triethoxysilane (KH550, chemical pure) and anatase nano-titanium dioxide (TiO2, diameter: 50 ± 10 nm) were purchased from the Aladdin Industrial Corporation (China).2.2 Synthesis
The experimental route for assembling nano-coatings is just like Fig. 1. Wood fibers and polypropylene were dried at 110 °C for 10 h to remove the moisture. In every program, under ultrasonic treatment, 16 g of wood fiber was soaked in 500 ml of KH550 solution (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silane
silane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 8
deionized water = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Nano-TiO2 particles were soaked in PEI solution (0.2 g, 0.4 g, 0.8 g, and 1.2 g in 500 ml of 1 wt% PEI solution) under ultrasonic conditions for 3 h. By making use of suction filtration, the final KH550-wood and PEI-nano-TiO2 were cleaned using absolute ethyl alcohol five times. Consequently, via adopting 16 g of wood fibers (grafted with KH550) and different ratios of TiO2 (grafted with PEI) under ultrasonic conditions, chemical assembly to construct the nano-coating occurred in a deionized water environment for 3 h. Furthermore, via particle analysis of the residual solution, the assembly was complete in every program.
1). Nano-TiO2 particles were soaked in PEI solution (0.2 g, 0.4 g, 0.8 g, and 1.2 g in 500 ml of 1 wt% PEI solution) under ultrasonic conditions for 3 h. By making use of suction filtration, the final KH550-wood and PEI-nano-TiO2 were cleaned using absolute ethyl alcohol five times. Consequently, via adopting 16 g of wood fibers (grafted with KH550) and different ratios of TiO2 (grafted with PEI) under ultrasonic conditions, chemical assembly to construct the nano-coating occurred in a deionized water environment for 3 h. Furthermore, via particle analysis of the residual solution, the assembly was complete in every program.
The wood–polypropylene composite with a chemically assembled nano-interface consisted of 40 wt% wood fibers (16 g) and 60 wt% polypropylene (24 g). Accordingly, the weight percent of the nano-TiO2 mentioned above was 0.5 wt%, 1.0 wt%, 2.0 wt%, and 3.0 wt%. In a torque rheometer, the synthesis temperature was fixed at 175 °C. To avoid wood decomposition, successful smelting occurred when the shearing interaction and the torque-curve value became stable. The final mixture was formed into a sheet shape using a plate rheometer at 180 °C under a uniaxial pressure of 5 MPa, 10 MPa, or 15 MPa per 3 min.
2.3 Functional characterization
The assembled morphology and fracture sections of the composites were observed under a sirion-200 scanning electron microscope (SEM, FEI, America) with an accelerating voltage of 10 kV. Structure analysis based chemical assembly was shown using an infrared absorption spectrometer (FTIR). The grafting influence on nano-crystallization and cellulose crystallization was shown using X-ray diffraction (XRD).Water absorption (mass fraction) and intensity features were evaluated five times. As in formula (1), this work used the same panel weight before soaking. Soaking was fixed at 23 °C (water temperature) under 101.3 kPa atmospheric conditions for 0 h, 6 h, 12 h, 24 h, 48 h, and 72 h.
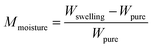 | (1) |
The light separation was measured using an ultraviolet-infrared spectrophotometer using a 1 mm plate sample. Relative analysis referred to the formula (2) and (3):
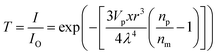 | (2) |
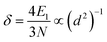 | (3) |
Tensile properties (GB/T 1040-92: 50 mm × 20 mm × 3 mm) and flexural properties (GB 1042-79: 55 mm × 6 mm × 4 mm) were determined using a CMT 4204 universal material testing system (Shenzhen SANS Test Machine Co., Ltd, China) at room temperature with a crosshead speed of 30 mm min−1. The values reported here represent an average of the results of ten tests. The influence on the viscoelasticity produced by the chemical assembly was measured using the dynamic mechanical tester (DMA, 20 mm × 6 mm × 1 mm) with a −50 °C to 160 °C temperature range. The protecting gas was N2 and the heat up rate was 1 °C min−1 at 2 Hz, and the output parameters were E′, E′′ and the loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ). The thermal decomposition process of the composites was analyzed using TG with the range of 50 °C to 800 °C by using N2 as the protecting gas.
δ). The thermal decomposition process of the composites was analyzed using TG with the range of 50 °C to 800 °C by using N2 as the protecting gas.
3. Results and discussion
3.1 Assembly of nano-TiO2-coating (wood-KH550–PEI-TiO2)
The experimental route is shown in Fig. 1. Cationic PEI, endowed with a high charge density, reacts with the hydroxyl groups of nano-TiO2. Subsequently, KH550-wood assembles PEI-TiO2 due to chemical interactions to construct a dispersive nano-coating on the wood surface.Fig. 2 shows the ZETA potentials and particle sizes of PEI-TiO2 in 500 ml of 1 wt% PEI solution. It can be clearly seen that the grafted PEI-TiO2 was endowed with a stable cationic potential, and the trend decreased with the increase of the TiO2 ratio due to the weakness of the efficient grafting area. When the amount of nano-TiO2 was 0.2 g (0.5 wt% of the WPCs), the highest zeta potential was 47.2 mV, representing the efficient charge grafting. Nano-particle size, an important aspect of nano-WPCs, determines the load transfer and interfacial adhesion largely. As can be seen, in Fig. 2(2), the nano-particle size was influenced obviously by charge repulsion, suggesting that the assembled nano-coating should be affected by the grafting effect. Compared with pure TiO2, the particle dispersion was apparently improved. However, due to the insufficient reaction of single particles and the incomplete charge intensity on the surface, the particle size with charge grafting still increased gradually with a higher ratio of TiO2. For the dispersion and the grafting effect, the results in Fig. 2 are consistent with the primary design.
 | ||
| Fig. 2 (1) Zeta potential of TiO2 with different ratios in 500 ml of 1 wt% PEI-solution; (2) particle size of TiO2 under different treatments. | ||
Fig. 3 shows the TEM images of PEI-TiO2. Compared with pure TiO2 (picture a1), PEI-grafting improved the dispersion to a certain extent in picture a2. Picture b1 shows the high-resolution image of PEI-TiO2 (101 crystal face, tetragonal system), which shows that the grafting effect had no apparent influence on the crystal features. Additionally, picture b2 gives the morphology of the grafted coating of PEI (low contrast), which is mainly formed at a thickness of 3–6 nm. Comprehensive results state that PEI-grafting, forming the reaction with single particles, is stable and sufficient.
 | ||
| Fig. 3 TEM images: (a1) 1.2 g TiO2 in 500 ml of deionized water; (a2) 1.2 g TiO2 in 500 ml of 1 wt% PEI solution; (b1) high-resolution image of a2; (b2) grafting morphology of a2. | ||
Wood structure, an important factor in determining the comprehensive properties of WPCs, was distinguished before and after assembly. The FTIR spectra showing the grafting effect are shown further in Fig. 4. In picture (1), especially, for KH550-wood, some peaks disappear, i.e., 1130 cm−1 (C–OH) and 1260 cm−1 (CH–OH), and some peaks weaken, for instance, 1520 (–NH), 1750 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2917 cm−1 (–CH3), and 3409 cm−1 (–OH, free). For chemical reactions, the results show that the wood fiber was grafted efficiently by KH-550. In picture (2), ascribed to the grafting, except for the weakness of –OH (3450 cm−1), peaks of 1510 cm−1 and 1550 cm−1 appear for PEI-TiO2, which demonstrates the existence of –NH2 and –NH efficiently.19 For the KH550-wood–PEI-TiO2 in picture (3), consistent with the assembly design, the newly formed peak at 1040 cm−1 (Si–N bond) shows the interaction between –NH and Si–OH. All of these illustrate that a chemical assembly to construct a nano-TiO2 coating on a wood surface is feasible, which could be applied to other active nano-particles.
O), 2917 cm−1 (–CH3), and 3409 cm−1 (–OH, free). For chemical reactions, the results show that the wood fiber was grafted efficiently by KH-550. In picture (2), ascribed to the grafting, except for the weakness of –OH (3450 cm−1), peaks of 1510 cm−1 and 1550 cm−1 appear for PEI-TiO2, which demonstrates the existence of –NH2 and –NH efficiently.19 For the KH550-wood–PEI-TiO2 in picture (3), consistent with the assembly design, the newly formed peak at 1040 cm−1 (Si–N bond) shows the interaction between –NH and Si–OH. All of these illustrate that a chemical assembly to construct a nano-TiO2 coating on a wood surface is feasible, which could be applied to other active nano-particles.
The morphology and micro-structure of the wood are shown further in Fig. 5. Compared with pure wood (picture a), wood with chemical nano-assemblies (pictures b–e) are adhered to tightly by dispersive nano-particles, which is attributed to the intense chemical activity and adhering properties of PEI and KH-550. However, for 1.2 g TiO2 on the surface (picture d), there are some aggregates on the surface, which is consistent with the size analysis in Fig. 3. In fact, layers on the wood surface, which are useful to construct a three dimensional nano-network in the WPC matrix, mainly consisted of three coatings: KH-550, PEI, and nano-TiO2. Except for the dispersive nano-coating structure, the organic chain features and grafting activity of PEI and KH-550 allowed the wood fibers to have a good compatibility with the polymer matrix, and thus could improve the homogeneity and mechanical strength at interfacial points.
With a high crystallinity, long polymer chain structure, and high mass fraction (∼50%), the wood strength is mainly dependent on cellulose, which was mainly observed in an I crystal form ((001) and (112), I crystal form).20 Anatase nano-TiO2 is a four-part crystal system, with a single cell consisting of four TiO2 molecules, and the coordination structure forms an n-type semiconductor. The relative diffraction peaks are concentrated on (110), (101), (200), (111), (210), (211), (220), (002), (310), (221), and (301).21 With the grafting of KH550 and PEI, in Fig. 6, it is worth noting that the crystal structure of nano-TiO2 and wood fiber was not influenced obviously, which means that the relative performance would be maintained well during the process of chemical assembly. Most importantly, in Fig. 6(3), wood with the nano-assembly was endowed with a multi-crystal structure, which explained the assembled structure in more detail.
 | ||
| Fig. 6 XRD spectra of (1) pure wood and KH550-wood; (2) pure TiO2 and PEI-TiO2; (3) KH550-wood–PEI-TiO2. | ||
3.2 Dimensional stability with the assembled nano-interface
By adapting the assembled nano-interface to reveal the possible influence on the hydrophobicity of the wood–polypropylene composite, Fig. 7 gives the features of water absorption by soaking for 6 h, 12 h, 24 h, 48 h, and 72 h. In general, the water absorption of WPCs should be due to mechanical interlocking and the secondary forces (dispersion, induced dipole, dipole–dipole, and H-bonds, etc.) between the polymer and the hydrophilic wood fillers. The trend in Fig. 7 shows that the absorbance increased if increasing the soaking time (from 0 h to 72 h), and the absorptive rate was faster in the initial period. Additionally, it is noted that the assembled nano-interface kept the composite firm in water when compared with the pure composite. The absorbance, after soaking for 6 h, only reached 0.72 wt% for the composite with the 2 wt% nano-interface. On the other hand, for the pure composite, after soaking for 6 h, the absorbance reached 2.89 wt%. The dispersive nano-TiO2 network is hydrophobic, so the dispersion and interactions of nano-particles determines the water resistance. The lower ratio does not have enough shielding effect, and the higher ratio does not have a good nano-dispersion to construct an efficient nano-network. On the other hand, the intensity features in Fig. 7 also state that the low absorbance reinforced the stable structure. For the assembled composite, the intensity trend is essentially flat although absorbing some moisture. The reason is that, different to the pure composite with an inflated construction, immersion in water had no apparent influence on the internal network of the composite with a compatible nano-interface.Ascribed to a three-dimensional segregated nano-network formed by chemical assembly, the results in Fig. 7 verify that the assembled nano-interface (about 0.5–3 wt%) is functional for hydrophobicity, which is an important factor in outdoor applications.22,23 In fact, except for a firm nano-network, according to wetting theory, a pure PEI-section decreased the shielding effect to a certain extent due to its hydrophilic features and high surface activity.
3.3 Ultraviolet-visible-near infrared resistance with an assembled nano-interface
Gong Lili and Su W. reported a UV-resistant composite which was based on TiO2 nano-structures.24,25 Nano-TiO2 has an absorption at 300–400 nm due to its active bonds and with about 300–400 kJ mol−1 energy can produce obvious electronic migrations in this range. By reference to the formula (2), ultraviolet transmittance is related to the nano-particle size and nano-particle concentration if the other parameters are constant, and a smaller size can increase the transmittance to a certain extent. As seen in Fig. 8, it is noted that ultraviolet absorption was improved via the nano-TiO2 network in the matrix. Compared with the pure composite, the absorptive peak moves to low wavelengths gradually when adopting the assembled interface. According to Kubo theory (formula (3)), the band gap is associated with particle size, and consequently the blue shift in Fig. 8 demonstrates the activity of nano-TiO2 in the matrix, suggesting that the nano-particle size and nano-particle activity of the chemical assembly are meaningful to ultraviolet shielding.Additionally, attributed to a dispersive nano-network, the assembled treatment increased the near infrared absorption to some extent while the reflection curves show the opposite trend. As mentioned above, the light absorption (transmittance) of the nano-composite is attributed largely to nano-particle dispersion and nano-particle size, and it can also be assumed that further research into the influence of different nano-particles with chemical assembly is meaningful in the light resistance of WPCs. In this work, chemical assembly provided the nano-TiO2 network with stability and good dispersion efficiency, which could be maintained well in the compounding process (melting and shearing). So the results in Fig. 8 repeatedly demonstrate that the assembled nano-TiO2 network, interlaced in the WPC matrix, is feasible for light separation.
3.4 Mechanical enhancements with assembled nano-interface
In general, there are four main requirements for effective reinforcements, including a large aspect ratio, good dispersion, alignment and interfacial stress transfer. Dispersion is probably the most fundamental issue for producing a more uniform stress distribution and minimum stress concentration. Table 1 shows the mechanical parameters of wood–polypropylene composites based on the assembled interface. Compared with pure composite, composites with the nano-interface have better tensile properties. Especially, when nano-TiO2 was 2.0 wt% in the composite, the tensile strength reached 22.92 MPa, which was enhanced by about 85%, and the elongation at break reached 23.91%, which was also improved. The reinforcements are similar to the reports by Esmaielzadeh and Zunic Vojka who indicated that a hydrothermal mechanism reinforced the matrix for efficient dispersion and a firm nano-network.26,27 For the structural aspect, the reinforcement of tensile strength should result from: (i) chemical grafting (coupling effect, H-bonding, and Van der Waals’ forces) weakening the wood activity and synchronously enhancing the sufficiency of the mechanical interlocking (mentioned above), and (ii) efficient nano-dispersion, as a level of isolated nano-particles grafted by PEI, building strong interfacial adhesion and a sufficient load transfer in matrix. The improvement of the elongation at break should be mainly attributed to the sufficient adhesive properties of the interface between wood and polypropylene. However, the adhesive interface has not improved the flexural properties of its weak anti-pressure ability, which has been counteracted to a certain extent by nano-activity.| Sample | Tensile properties | Flexural properties | ||||
|---|---|---|---|---|---|---|
| Tensile strength (Mpa) | Elastic modulus (Mpa) | Elongation at break (%) | Flexural strength (Mpa) | Flexural modulus (Mpa) | Largest flexural force (N) | |
| Pure composite | 12.38 ± 0.32 | 139.30 ± 6.60 | 10.61 ± 0.19 | 52.21 ± 2.89 | 668.40 ± 42.6 | 51.56 ± 2.17 |
| Composite-0.5 wt% | 16.32 ± 0.68 | 155.10 ± 7.48 | 19.66 ± 2.89 | 43.88 ± 4.31 | 598.60 ± 36.3 | 44.45 ± 4.89 |
| Composite-1 wt% | 17.92 ± 1.8 | 163.32 ± 12.80 | 21.24 ± 2.18 | 51.82 ± 3.89 | 812.70 ± 43.8 | 52.78 ± 4.60 |
| Composite-2 wt% | 22.92 ± 1.69 | 198.30 ± 13.20 | 23.91 ± 4.39 | 58.69 ± 6.65 | 1016.60 ± 66.2 | 62.85 ± 4.99 |
| Composite-3 wt% | 19.67 ± 2.1 | 178.80 ± 12.39 | 20.51 ± 5.18 | 51.28 ± 4.28 | 982.50 ± 58.9 | 53.92 ± 6.23 |
In Fig. 9, the viscoelastic properties are shown further. The results state that molecular flowing became easy with an increase of temperature. The storage reached the optimal value when the nano-TiO2 was about 2 wt%. However, the loss factor produced the opposite trend. The peaks at about 11–23 °C, representing the glass transition temperature (Tg) of polypropylene, increased in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ spectra when with the assembled nano-interface, and it reached 23.2 °C for the composite with 2.0 wt% nano-TiO2 while the same of the pure composite was only 16.8 °C. Except for surface activity and intense interactions between the active nano-particles and chain segments, the adhesive interface also hindered the motion of the chain segments. Furthermore, the SEM images of the tensile fracture sections are shown in Fig. 10. As predicted above, different to the pure composite with a separation structure, nano-dispersion and homogeneity are good in pictures (b), (c), and (d). However, in picture (f), there are some aggregates in the composite with a 3.0 wt% nano-interface, which is likely due to the larger nano-particle size and the weak interfacial reaction. The results illustrate that the nano-particle assembly, constructing the uniform nano-particle dispersion and the strong interfacial interactions between nano-TiO2 and the WPC matrix, is controlled as predicted.
δ spectra when with the assembled nano-interface, and it reached 23.2 °C for the composite with 2.0 wt% nano-TiO2 while the same of the pure composite was only 16.8 °C. Except for surface activity and intense interactions between the active nano-particles and chain segments, the adhesive interface also hindered the motion of the chain segments. Furthermore, the SEM images of the tensile fracture sections are shown in Fig. 10. As predicted above, different to the pure composite with a separation structure, nano-dispersion and homogeneity are good in pictures (b), (c), and (d). However, in picture (f), there are some aggregates in the composite with a 3.0 wt% nano-interface, which is likely due to the larger nano-particle size and the weak interfacial reaction. The results illustrate that the nano-particle assembly, constructing the uniform nano-particle dispersion and the strong interfacial interactions between nano-TiO2 and the WPC matrix, is controlled as predicted.
3.5 Thermal shielding with the assembled nano-interface
In this work, the wood–polypropylene composite has two characteristic decomposition (Td) stages. The first is wood decomposition which occurs at about 207 °C, and the second step is mainly the polypropylene decomposition which occurs at about 375 °C.28 Via chemical assembly, the Td features are shown in Fig. 11. Compared with the pure composite, the composites with the assembled interface have a higher value in these two steps. When the assembled-TiO2 was 3.0 wt%, the wood decomposition and polypropylene decomposition reached 286 °C and 402 °C, respectively. To explore the grafting ratio for KH550–PEI, this work compared the weight loss of the pure composite and the composite with the 0.5 wt% nano-assembled interface. After fitting, the 4.3 wt% difference reflects that the grafting ratio of the KH550–PEI of composite is nearly 4.9 wt%, showing the sufficient assembly on the wood surface.The results in Fig. 11 reflect two points. The first is that the three-dimensional nano-network was constructed well in the assembling process, and the interfacial-TiO2 transferred the heat effectively. The network hindered the diffusion of degradation components from the matrix to the gaseous phase efficiently. The second is that the active coupling effect between PEI-TiO2 and KH550-wood, promoting the polypropylene-separation for wood, increased the difficulty of the wood decomposition to a certain extent. Thus, the chemical assembly to construct a three-dimensional nano-network in recycled WPCs is functional in thermal shielding, which can be further researched by using heat-resistant fillers to guide applications in flame retarding and high-temperature WPCs.
4. Conclusions
Chemical assembly to construct a compatible nano-TiO2-interface with polyethyleneimine and (3-aminopropyl) triethoxysilane was studied in recycled WPCs. The crystal features, ZETA potential, and FTIR analysis showed the assembling effect was chemically stable and sufficient, which could be demonstrated further by the SEM images. Compared with the pure composite, the properties of dimensional stability, separation in ultraviolet-visible-near-infrared light, mechanical reinforcements, and thermal shielding are reinforced well with the chemical nano-assembly. Via a three-dimensional hydrophobic network, consistent with the density trend, the absorbance decreased from 2.89 wt% to 0.72 wt%. Ultraviolet and near-infrared resistance were enhanced obviously for the dispersive nano-network in the composite matrix. In particular, for the strong interfacial adhesion and sufficient load transfer, the tensile strength was enhanced by about 85%, which could be further observed by Tg features. Additionally, by the shielding and the coupling effect of the nano-interface in WPCs, the Td of wood and polypropylene was enhanced by about 79 °C and 27 °C, respectively.Acknowledgements
The authors are grateful to the support of the Anhui Provincial Natural Science Foundation (1508085QE112, 1508085QE110) and the Youth Innovation Promotion Association, CAS (2015268).References
- D. Zita, D. Livia and P. Bela, Composites, Part A, 2007, 38, 1893–1901 CrossRef
.
- M. Hietala, J. Niinimaki and K. Oksman, Plast., Rubber Compos., 2011, 40, 49–56 CrossRef CAS
.
- C. Yihua, L. Stephen and N. Bahman, Composites, Part A, 2008, 39, 655–661 CrossRef
.
- T. Irina and K. Timo, Eur. J. Wood Wood Prod., 2014, 72, 73–79 CrossRef
.
- S. Houssine, A. Mael and Z. Qi, Compos. Sci. Technol., 2011, 71, 382–387 CrossRef
.
- G. Zhengxin, M. Miaolian and Z. Xianglin, RSC Adv., 2015, 5, 63978–63984 RSC
.
- L. Zhiyuan and R. Scott, Composites, Part A, 2011, 42, 84–91 CrossRef
.
- X. Liaoyuan, L. Xianjun and W. Yiqiang, ACS Sustainable Chem. Eng., 2015, 3, 1724–1731 CrossRef
.
- Z. Yongcheng, X. Yibin and T. Hossein, Compos. Interfaces, 2009, 16, 671–686 CrossRef
.
- X. Cheng, Q. Rongrong and G. Wenjuan, Polym. Adv. Technol., 2009, 20, 273–279 CrossRef
.
- L. Yong and W. Qinglin, Composites, Part A, 2012, 43, 73–78 CrossRef
.
- L. Zewen, G. Hua and W. Qingwen, J. Appl. Polym. Sci., 2012, 124, 5247–5253 Search PubMed
.
- Z. Michaela, K. Daniel and M. Rajmund, Holzforschung, 2012, 66, 973–979 Search PubMed
.
- M. Shichun, W. Xiaoen and T. Haolin, J. Electrochem. Soc., 2006, 153, A1868–A1872 CrossRef
.
- T. Hao Lin and P. Mu, J. Phys. Chem. C, 2008, 112, 11556–11568 Search PubMed
.
- M. Agarwal, Q. Xing, B. S. Shim, N. Kotov, K. Varahramyan and Y. Lvov, Nanotechnology, 2009, 20, 215602 CrossRef PubMed
.
- C. Yufei, Y. Wei and B. Zongzhen, Iran. Polym. J., 2013, 22, 377–383 CrossRef
.
- W. Bigui, C. Qing and B. Caixia, Colloids Surf., A, 2013, 434, 276–280 CrossRef
.
- Y. Tohi, T. Nakano, H. Makio, S. Matsui, T. Fujita and T. Yamaguchi, Macromol. Chem. Phys., 2004, 205, 1179–1186 CrossRef CAS
.
- G. Chauvelon, L. Saulnier and A. Buleon, J. Appl. Polym. Sci., 1999, 74, 1933–1940 CrossRef CAS
.
- Z. Rongbo, A. Tshabalala Mandla and L. Qingyu, Appl. Surf. Sci., 2015, 328, 453–458 CrossRef
.
- N. Saeed Kazemi, K. Azadeh and H. Elham, J. Reinf. Plast. Compos., 2007, 26, 341–348 CrossRef
.
- W. Xu, P. M. Winistorfer and W. W. Moschler, Wood Fiber Sci., 1996, 28, 286–294 CAS
.
- G. Lili, Z. Lei and W. Naixin, Sep. Purif. Technol., 2014, 122, 32–40 CrossRef
.
- W. Su, S. S. Wei and S. Q. Hu, J. Hazard. Mater., 2009, 172, 716–720 CrossRef CAS PubMed
.
- M. R. Vaezi and K. A. Esmaielzadeh, J. Ceram. Process. Res., 2014, 15, 376–381 Search PubMed
.
- Z. Vojka, V. Marija and D. Skapin Sreco, Ultrason. Sonochem., 2014, 21, 367–375 CrossRef PubMed
.
- W. Chou, C. Wang and C. Chen, Compos. Sci. Technol., 2008, 68, 2208–2213 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |








