DOI:
10.1039/C5RA21502A
(Paper)
RSC Adv., 2016,
6, 19078-19088
A new efficient technology for refractory phenol-formaldehyde resin wastewater treatment†
Received
15th October 2015
, Accepted 5th February 2016
First published on 5th February 2016
Abstract
Phenol-formaldehyde resin wastewater contains high concentrations of phenol and formaldehyde, which have strong bio-resistance and toxicity to microbes, thus limiting the direct use of biological treatment methods. At present, studies on phenol-formaldehyde resin wastewater treatment are very few. The main objective of this study was to systematically evaluate the feasibility of O3/MgO/H2O2 – coagulation–mechanical membrane filtration process as a pretreatment method for such wastewater. The influence of several factors – including dosage of MgO powder as well as the concentrations of H2O2, O3, polyaluminum chloride (PAC) and cationic polyacrylamide (CPAM) – was investigated. Experimental results indicated the optimal operational conditions were a 6 g L−1 dose of MgO powder, 5 g L−1 concentration of H2O2, 0.25 g L−1 min−1 dose of O3, 3 g L−1 of PAC, 30 mg L−1 of CPAM and use of a four-stage mechanical membrane. The concentrations of phenol and formaldehyde dropped to 28 mg L−1 and 43 mg L−1, respectively from the initial 4790 mg L−1 and 2660 mg L−1. The corresponding removal ratios were as high as 99.4% and 98.3%, respectively. Also, the residual turbidity was under 2.0 NTU and suspended solids (SS) could not be detected. The ratio of biochemical oxygen demand and chemical oxygen demand (BOD/COD) increased from 0.165 to 0.364, indicating a good biodegradability. The effluent satisfied the requirements for direct access to the biochemical tank. Therefore, the combined process is a promising technology and may be used in the pretreatment of industrial phenol-formaldehyde resin wastewater.
1. Introduction
Phenol-formaldehyde resin is synthesized from phenol and formaldehyde with the assistance of a catalyst under acid or alkaline conditions. Wastewater generated by phenol-formaldehyde resin producing industries contains high concentrations of phenol, formaldehyde and free small resin and has been reported to exert high toxicity on the environment and human health because of its carcinogenicity.1 These wastewaters have high concentrations of organic matter – 40 × 103 mg L−1 of COD and/or between 2 × 104 and 3 × 104 mg L−1 of total organic carbon (TOC),2 high viscosity, strong corrosivity, poor biodegradability and active chemical property. For wastewater containing concentrated COD, biological processes enjoy the priority at home and abroad because they can completely mineralize the contaminants with economic advantages. However, the microorganisms, even well acclimated, can only dispose of wastewater containing low concentrations of phenol, usually less than 100 mg L−1.3 In addition, formaldehyde inhibits microbial activity at concentrations higher than 250 mg L−1.4 At present, these wastewaters are diluted before being discharged for biological water treatment or are incinerated,5 which not only demands plentiful clean water, but also contaminates both water bodies and the atmosphere. Several methods have been reported in the literature for the treatment of wastewater containing phenol or formaldehyde compounds.3,6–11 However, studies on specialized phenol-formaldehyde resin wastewater treatment are very few.
In this study, the ultimate goal was to improve the biodegradability of such wastewater by greatly reducing the concentrations of phenol and formaldehyde to a level that is tolerable for microorganisms. To meet the requirements of the subsequent biological treatment, the value of BOD/COD, as a judgement for biodegradability, should be higher than 0.3. Also, the complete removal of SS and turbidity was necessary. For treatment of wastewater with high phenol or formaldehyde, advanced oxidation processes (AOPs) including ozonation, photocatalysis, electrolytic oxidation and Fenton have been employed as alternative pretreatments for biological process due to their high degradation efficiency, simple application and ability to function at normal temperature and pressure.12,13 In AOP, highly reactive radicals are generated, mainly hydroxyl radical (˙OH),14,15 which has a redox potential of 2.80 eV and a strong ability to oxidize organic matter into inorganic final products and/or less toxic small molecule intermediates, thus reducing inhibition of subsequent biological systems.16 Among the AOPs, ozone is widely used as the principal component. The efficient integration of ozonation with MgO nanocrystals has been used for the removal of phenol.17 Moussavi et al.9 showed the feasibility of an AOP-based process – a more efficient catalytic advanced oxidation process (CAOP) utilizing O3/MgO/H2O2 for degradation of high formaldehyde wastewater, which obtained better performance in comparison with several other oxidation processes, including single ozonation, O3/MgO, MgO/H2O2, O3/H2O2 and O3/TiO2/H2O2. The coagulation–membrane filtration process, as an efficient water treatment technology, has wide application.18,19 This process showed great talent for complete removal of turbidity and SS. However, after a period of operation, it would cause membrane fouling and clogging, leading to serious flux decline and poor water quality. By contrast, the mechanical membrane showed low fouling tendency because of the triangular filament gaps and smooth surface, extensively reducing operation costs.20 Also, it can be easily recycled by reverse flushing with clean water, after which recovery efficiency of water flux can reach more than 95%.20 Among many common coagulants, PAC showed its superiority because of its high efficiency and low cost.21 Some studies have shown that the addition of polymer flocculants can improve dissolved organic carbon (DOC) removal efficiency in conjunction with metal salt primary coagulants.22 Moreover, it has been reported that high flux was greatly extended with CPAM (107 Da) at a dose of 0.3 mg L−1.23
In the present work, CAOP of O3/MgO/H2O2 coupled with a coagulation–mechanical membrane filtration process was developed to treat phenol-formaldehyde resin wastewater and the feasibility was demonstrated. Different operational variables – including dosages of H2O2, O3, MgO, PAC, CPAM – were investigated to determine the optimal operational conditions, in which the highest removal efficiency of turbidity, SS, BOD, COD, phenol and formaldehyde as well as high value of BOD/COD were achieved. Furthermore, the performance of a self-prepared mechanical membrane module was explored for the subsequent treatment of the effluent from the coagulation process under optimum conditions.
2. Materials and methods
2.1. Materials and chemicals
Chemicals used in this study were all analytical pure except for the chromatographic grade normal hexane (YuWang Group). PAC with Al2O3 content of 28% (w/w) (Dingshengxin, Tianjin) was used as primary flocculant in all jar tests. The CPAM of 4000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa used as polymer coagulant was purchased from Fuyuan Water Reagent Co., LTD. Formaldehyde-2,4-dinitrophenylhydrazone (FA-DNPH) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Other chemicals were supplied by Sinopharm Chemical Reagent Co., LTD. All solutions were prepared with deionized water. 1 g L−1 of 2,4-dinitrophenylhydrazine (DNPH) solution was prepared by dissolving 0.1 g DNPH into 25.0 mL HCl, followed by being diluted to 100 mL with deionized water. Formaldehyde standard solution (5 g L−1) was roughly prepared by diluting 36.0–38.0% formaldehyde solution with deionized water and then its accurate concentration was determined using the iodometric method.24 The powdered MgO nanocrystals were prepared by calcining a Mg(NO3)2 solution and the detailed production procedure is given elsewhere.25 Organic glass cylinder and copper filaments were used to prepare the mechanical membrane. Small pores with a diameter of 0.3 mm were punched uniformly on the surface of organic glass, which has a height of 20 cm and a thickness of 1 mm. On the basis of the technology in patent ZL 200810140398.3, four cylindrical mechanical membranes with different layers and diameters numbered 1, 2, 3 and 4, respectively, were successfully prepared, whose total filtration area was 344.9 cm2, 460.0 cm2, 574.9 cm2 and 689.9 cm2, respectively.
000 kDa used as polymer coagulant was purchased from Fuyuan Water Reagent Co., LTD. Formaldehyde-2,4-dinitrophenylhydrazone (FA-DNPH) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Other chemicals were supplied by Sinopharm Chemical Reagent Co., LTD. All solutions were prepared with deionized water. 1 g L−1 of 2,4-dinitrophenylhydrazine (DNPH) solution was prepared by dissolving 0.1 g DNPH into 25.0 mL HCl, followed by being diluted to 100 mL with deionized water. Formaldehyde standard solution (5 g L−1) was roughly prepared by diluting 36.0–38.0% formaldehyde solution with deionized water and then its accurate concentration was determined using the iodometric method.24 The powdered MgO nanocrystals were prepared by calcining a Mg(NO3)2 solution and the detailed production procedure is given elsewhere.25 Organic glass cylinder and copper filaments were used to prepare the mechanical membrane. Small pores with a diameter of 0.3 mm were punched uniformly on the surface of organic glass, which has a height of 20 cm and a thickness of 1 mm. On the basis of the technology in patent ZL 200810140398.3, four cylindrical mechanical membranes with different layers and diameters numbered 1, 2, 3 and 4, respectively, were successfully prepared, whose total filtration area was 344.9 cm2, 460.0 cm2, 574.9 cm2 and 689.9 cm2, respectively.
2.2. Experimental apparatus and procedure
The experimental setup contained a jar tester, an ozone generator, a gas diffuser for distributing ozone to the solution, an oxygen cylinder equipped with flowmeter and control valve, and a self-developed multi-stage mechanical membrane reactor with an effective volume of 5 L. Ozone was produced from oxygen (99.999%, Deyang Co., LTD, Jinan, China) by a laboratory generator (SK-CFG-5, Sankang environmental protection technology Co., LTD, Jinan, China). The ozone dose was regulated by changing the flow rate of feed oxygen. CAOP and coagulation trials were conducted at a room temperature of 25 ± 2 °C on a MY3000-6B jar tester (Meiyu Instruments Co., Ltd, Wuhan, China) equipped with six cylindrical jars containing 1 L of water samples. According the results of previous works,9,17 the pH of the water samples was adjusted to 8 using NaOH (0.1 N) and then H2O2, MgO and O3 were added at a stirrer speed of 60 rpm. After 120 minutes of oxidation, PAC was added at the very beginning of the rapid stage at 200 rpm, followed by CPAM after a 30 s interval. Then rapid stirring was continued for 60 s, followed by a slow flocculation stage at 40 rpm for 20 min. Mechanical membrane filtration of the coagulation effluent was carried out in a bench scale organic glass reactor with an inner diameter of 15 cm and an effective height of 28 cm, giving a working volume of 5 L. It has an inlet on the top and four outlets on the bottom, which was equipped with four card slots for assembling the four mechanical membranes. Water samples were carried by LEAD-1 peristaltic pump (Longer Precision Pump, China) at a flow rate of 2.0 L h−1 from the outlet of the jar tester after 30 minutes of quiescent settling. To observe the antifouling ability of the membrane, the filtration was run continuously for a total of 300 h. The seriously contaminated membrane was recycled by reverse flushing with clean water. The detailed schematic on the entire treatment is shown in Fig. 1.
 |
| | Fig. 1 Schematic diagram of the combined CAOP–coagulation–mechanical membrane filtration process. | |
2.3. Analytical methods
The self-prepared membranes were characterized for average and maximum pore size, porosity, permeation flux and thickness. For pore size determination, the bubble-pressure method was employed, where the membrane was fully wet by water and then a differential pressure was imposed by nitrogen. Pure water flux was measured at 25 °C under a pressure of 0.1 MPa to determine the membrane permeability. Size of pores, porosity, water flux and thickness were calculated as follows:| | |
ε = (m1 − m2)/ρH2OV1 × 100%
| (2) |
here, σ is the surface tension of water, ΔP is the differential pressure, ε is the porosity, m1 is the mass of wet film, m2 is the mass of dry film, ρH2O is the water density, V1 is the apparent volume of membrane, J is the water flux; V is the water volume through membrane within a certain time, S is membrane area, t is operation time and ρ is the density of filament material.
The samples were analyzed for turbidity, SS, BOD, COD and pH as well as concentration of phenol and formaldehyde. Before analysis, the residual ozone dissolved in water was destroyed by adding 1 mL (1 N) sodium thiosulfate solution. Turbidity and pH were determined using a WGZ-200 turbidimeter (Shanghai Instrument Physical Optics Instrument Co., LTD, China) and a PXS-215 pH meter (Shanghai Ridao Co., LTD, China), respectively. BOD was determined using the dilution and seeding method, COD was measured using the standard potassium dichromate oxidation method and SS was analyzed with the gravimetric method after filtration through a 0.45 μm microfiber membrane.26 Phenol and formaldehyde concentration were determined by GC. The analysis procedure of phenol and formaldehyde is presented in the next section.
2.4. Analysis of phenol and formaldehyde
The analysis involved three parts, including derivatization, solvent extraction and GC determination. For derivatization, 1 mL water sample was transferred into colorimetric tube, where 25 mL DNPH with a concentration of 1 g L−1 was added. The colorimetric tube was placed in water bath for 20 min at 65 °C to form the derivative. Afterwards, the solution was cooled down with cold water. Then 5 mL of chloroform was appended to test tube, followed immediately by ultrasonic oscillation for five minutes. When the derivation and extraction were complete, the organic phase was separated from the aqueous phase and then dried by adding anhydrous sodium sulfate. GC-2014 (Shimadzu, Japan) with a flame ionization detector (FID) and an AOC-20i split/splitless auto injector (Shimadzu) was applied for the determination of phenol and formaldehyde. The GC-FID was equipped with an Rtx-5 (5% diphenyl, 95% dimethyl polysiloxane) capillary column (30 m × 0.25 mm i.d., 0.25 μm) from Restek (USA). Nitrogen (99.999%, Deyang Co., LTD, Jinan, China) was used as the carrier gas with a flow rate of 1.0 mL min−1. The injector temperature was set at 260 °C and all injection volumes were 0.5 μL with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 split ratio. The column temperature was initially maintained at 100 °C for 4 min, and then temperature increased up to 250 °C at a rate of 50 °C min−1 and finally this temperature was held for 5 min. The total running time for each sample was 12 min. The FID temperature was set at 280 °C with a H2 flow of 30 mL min−1 and an air flow of 300 mL min−1.
30 split ratio. The column temperature was initially maintained at 100 °C for 4 min, and then temperature increased up to 250 °C at a rate of 50 °C min−1 and finally this temperature was held for 5 min. The total running time for each sample was 12 min. The FID temperature was set at 280 °C with a H2 flow of 30 mL min−1 and an air flow of 300 mL min−1.
2.5. Raw water
Water samples used in this study were collected in plastic containers from the outlet of the phenol-formaldehyde resin production workshop of Jinan Shengquan Co., LTD. In order to reduce the decomposition of phenol by microbes in water, it should be carefully stored in dark at 4 °C with the addition of a moderate amount of copper sulfate. Physicochemical parameters of the test water are shown in Table 1.
Table 1 Phenol-formaldehyde resin wastewater characteristics
| Parameter |
Value |
| pH |
2.13 ± 0.02 |
| Turbidity |
428.0 ± 20.0 NTU |
| SS |
550.0 ± 10.0 mg L−1 |
| BOD |
2990 ± 150.0 mg L−1 |
| COD |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 ± 1650 mg L−1 995 ± 1650 mg L−1 |
| BOD/COD |
0.151 ± 0.020 |
| Phenol |
4790.0 ± 170.0 mg L−1 |
| Formaldehyde |
2660.0 ± 140.0 mg L−1 |
3. Results and discussion
3.1. Mechanical membrane characteristics
Characteristics of self-prepared membranes were observed and are summarized in Table 2.
Table 2 Characteristics of the 4 different membranes
| Parameter |
Value |
| Membrane number |
1 |
2 |
3 |
4 |
| Average pore size (μm) |
92 |
38.8 |
15.7 |
6.2 |
| Maximum pore size (μm) |
107 |
49.6 |
21.8 |
10.1 |
| Porosity (%) |
31.88 |
26.81 |
20.06 |
18.16 |
| Permeation flux (mL cm−2 h−1) |
418.42 |
170.16 |
51.57 |
12.73 |
| Thickness (mm) |
0.22 |
0.42 |
0.60 |
0.75 |
3.2. Factors influencing CAOP performance
3.2.1. Effects of H2O2 concentration on O3/MgO/H2O2 process. The dosage of ozone and MgO powder were set at 0.25 g L−1 min−1 and 5 g L−1, respectively. The influence of the H2O2 concentration (ranging between 0 and 10 g L−1) on the CAOP (O3/MgO/H2O2) performance is shown in Fig. 2. The removal ratios of turbidity and SS were much lower than 10%, thus they are not presented in the following figure. As shown in Fig. 2, removal rates improved rapidly with an increase of H2O2 concentration, up to 6 g L−1 and the removal efficiencies of BOD, COD, phenol and formaldehyde were 36.8%, 72.2%, 91.8% and 86.1%, respectively. BOD/COD increased to 0.34 from the initial 0.16. The good performance can be interpreted as an enhanced formation of various types of oxidizing radicals with a higher concentration of H2O2,14,15,27 involving (MgO-sO˙), (MgO-s˙OH) and free ˙OH, which took part in the indirect radical-type oxidation. The mechanisms of the radical's formation are proposed as follows9:| | |
O3 + (MgO-s) → (MgO-sO3)
| (5) |
| | |
(MgO-sO3) → (MgO-sO˙) + O2
| (6) |
| | |
(MgO-sO˙) + 2H2O2 + O3 → (MgO-s˙OH) + 3˙OH + 2O2
| (7) |
| | |
H2O2 + 2O3 → 2˙OH + 3O2
| (8) |
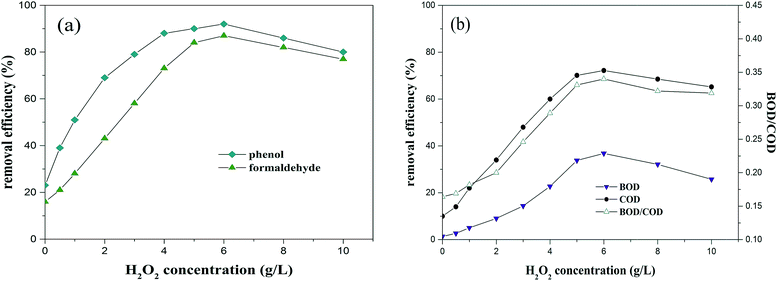 |
| | Fig. 2 Effect of the H2O2 concentration on the CAOP performance (experimental conditions: MgO dose 5 g L−1, O3 concentration 0.25 g L−1 min−1, pH 8 and reaction time 120 min). | |
The suffix s in MgO-s is on behalf of the Lewis acid site on the surface of MgO.
The predominance of radical-type oxidation in the CAOP (O3/MgO/H2O2) process was confirmed by the different results in the presence and/or absence of the well-known radical scavenger tert-butanol.9 However, a further increase of the H2O2 concentration resulted in a reduction in the removal efficiency, indicating excess H2O2 inhibited the removal. There are two possible explanations, one is H2O2 serves as a radical scavenger and/or inhibitor28,29 and the other is when excess H2O2 is added, less reactive radicals, such as hydroperoxyl radical are generated.30 From Fig. 2, it can be found a highly efficient O3/MgO/H2O2 process requires a proper H2O2 concentration, which depends on the pollutant type and concentration as well as the operating conditions.28,30
3.2.2. Effects of MgO dosage on O3/MgO/H2O2 performance. The effects of different concentrations of MgO nanocrystals (0 to 8 g L−1) on the performance of CAOP are presented in Fig. 3. Experiments were performed with a H2O2 dosage of 5 g L−1 and O3 concentration of 0.25 g L−1 min−1. As indicated in Fig. 3, all the removal percentages were directly enhanced with the increase of MgO dosage. Removal ratios of phenol, formaldehyde, BOD and COD increased from 15%, 12%, 1% and 9% respectively without MgO to 76%, 62%, 16.4% and 46% in the presence of 2 g L−1 MgO powder, revealing a significant catalytic effect for the O3/H2O2 process. When 8 g L−1 of MgO was added, removal ratios reached 93.5%, 89.1%, 37.9% and 74.5%. The catalytic effect of MgO may be due to its polar surface, which showed good ability of adsorption and subsequent decomposition of polar O3 molecules. Therefore, a larger dosage of MgO can provide more available catalyst surface as well as active sites for decomposing ozone, followed by more generation of reactive radicals, leading to higher removal ratios.
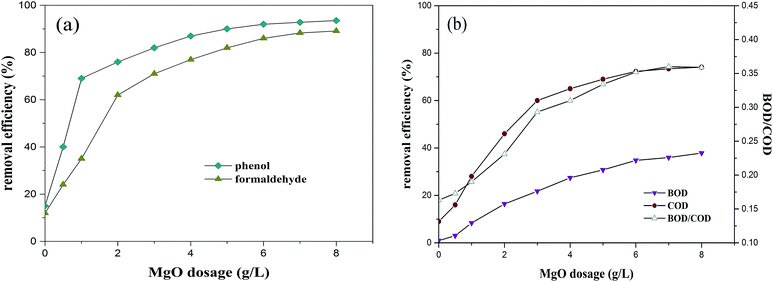 |
| | Fig. 3 Effect of MgO dosage on the CAOP performance (experimental conditions: H2O2 concentration 5 g L−1, O3 concentration 0.25 g L−1 min−1, pH 8 and reaction time 120 min). | |
Previous report17 proved the MgO powder an efficient and promising catalyst for ozonation to remove phenol from saline wastewater. Moussavi et al.9 reported that adding 5 g L−1 catalytic MgO nanocrystals to concentrated formaldehyde wastewater greatly improved the O3/H2O2 performance and the oxidation efficiency was positively correlated with the catalyst dosage. Other researchers have noticed the same tendency in other metal-based AOPs.31,32
3.2.3. Effect of O3 concentration on the O3/MgO/H2O2 process. MgO dosage of 6 g L−1 and H2O2 concentration of 5 g L−1 were chosen to carry out the following studies. The O3/MgO/H2O2 performance as a function of O3 (ranging from 0 to 0.30 g L−1 min−1) is illustrated in Fig. 4. It can be seen that the removal efficiency of phenol and formaldehyde increased from 18% and 15% to 79.1% and 62.3% when the concentration of O3 increased from 0 to 0.1 g L−1 min−1. Thereafter, an approximately linear relationship was observed between formaldehyde as well as phenol removal and the O3 dosage in the range from 0.10 to 0.25 g L−1 min−1, followed by a gentle increase. Lei et al.33 observed a higher removal percentage with increasing O3 dosage in a catalytic ozonation with activated carbon. Since O3 is the initiator for radical generation, higher concentration could supply more available O3 to contribute to the reaction and promote the formation of more radicals,34 resulting in a better CAOP performance. Another explanation for the removal efficiency being positively related to the O3 dosage might be due to an increased turbulence in the suspension caused by the increase of ozone flow rate, and in return accelerated diffusion and mass transfer of O3 into the liquid phase.35,36
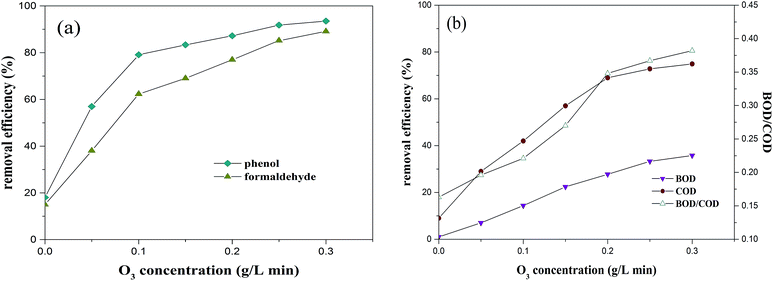 |
| | Fig. 4 Effect of the O3 concentration on the CAOP performance (experimental conditions: H2O2 concentration 5 g L−1, MgO dose 6 g L−1, pH 8 and reaction time 120 min). | |
3.3. Effects of PAC dosage on coagulation effects
Based on Section 3.2, the concentrations of MgO, H2O2 and O3 were set to 6 g L−1, 5 g L−1 and 0.25 g L−1 min−1, respectively in the following experiments. pH after CAOP was 6.50 ± 0.10, under which PAC had a good coagulation performance.37 Removal efficiencies as a function of dual-coagulant concentration after 30 minutes of sedimentation were tested (Fig. 5a–e).
 |
| | Fig. 5 Effects of dosage of PAC and CPAM on coagulation performance. (a) Turbidity removal efficiency; (b) SS removal efficiency; (c) BOD removal efficiency; (d) COD removal efficiency; (e) phenol removal efficiency; (f) formaldehyde removal efficiency. | |
When PAC was dosed alone, the minimum residual turbidity was obtained when 3 g L−1 PAC was added and others had the best performance with a PAC dosage of 4 g L−1. Removal efficiencies increased sharply with the increase of PAC dosage and then declined rapidly when PAC concentration exceeded the optimal point. This was because that under neutral conditions, most aluminum species of PAC were hydrolyzed to a series of positively charged monomer ions, such as Al3+, Al(OH)2+ and Al(OH)2+, which later formed polymeric species and integrated with negatively charged colloid particles in the water sample to destabilize the colloid particles and produce co-precipitates.38,39 When PAC dosage was low, the coagulation efficiencies were lower for only limited positively charged ions were produced to participate in the neutralization reaction. With further increase of PAC concentration, excess positively charged ions were absorbed on the surface of precipitates and caused the colloid particles to stabilize again by the electrostatic repulsions between them.38 As a consequence, the coagulation performance was reduced at higher dosages.
With the addition of CPAM, the coagulation performance with flocculant dosage showed the same tendency as that of PAC. But it showed a significant improvement of coagulation performance compared with PAC. The maximum removal was achieved with a PAC dosage of 3 g L−1 and a CPAM dosage of 30 mg L−1, under which removal efficiencies for turbidity, SS, BOD, COD, phenol and formaldehyde were 86.0%, 75.4%, 67.4%, 87.2%, 98.3% and 96.4%, much higher than when PAC was added alone. This was because that as organic macromolecular polymer, the long-chain branched CPAM showed a good ability to absorb the small floccules and bridge them, causing the size of floc particles to enlarge and the coagulation efficiencies to rise.40 In addition, as a cationic flocculant, CPAM can help to neutralize the negative charge on the surface and eliminate the electrostatic repulsion.41 With the continuous increase of CPAM dosage, the polymer in demand gradually tended to become saturated. In addition, excess CPAM can cause pollution, leading to a drop in COD and BOD removal. The results indicated that the addition of CPAM can not only reduce PAC dosage to lower the cost, but also greatly improve the coagulation performance.
3.4. Studies on removal effects of mechanical membrane module
Water qualities after coagulation are summarized in Table 3.
Table 3 Water qualities after coagulation
| Parameter |
Value |
| pH |
5.54 ± 0.05 |
| Turbidity |
53.0 ± 2.5 NTU |
| SS |
135.0 ± 5.0 mg L−1 |
| BOD |
975 ± 50.0 mg L−1 |
| COD |
2560 ± 215 mg L−1 |
| BOD/COD |
0.351 ± 0.018 |
| Phenol |
80.0 ± 2.8 mg L−1 |
| Formaldehyde |
95.0 ± 5.0 mg L−1 |
3.4.1. Single-stage membrane filtration experiments. In order to investigate the performance of each membrane module, water samples were obtained from the corresponding outlet for quality and flux determination. Residual turbidity, SS, COD, BOD, phenol and formaldehyde levels over operating time are listed in Fig. 6a–e. The changes of water flux are illustrated in Fig. 7a–d.
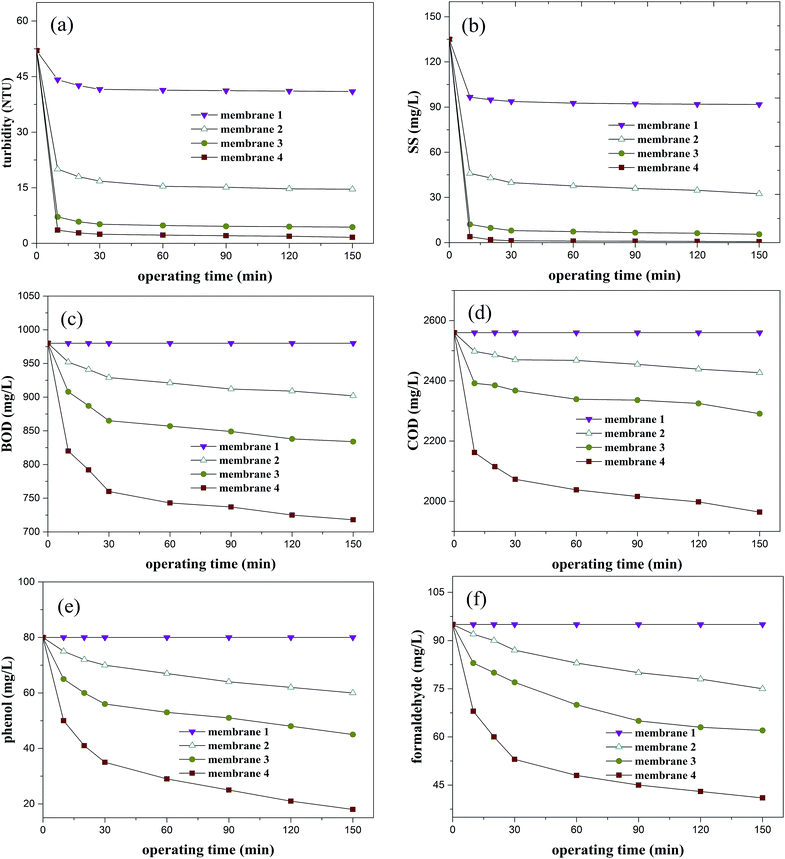 |
| | Fig. 6 Effects of operating time on filtration performance. (a) Turbidity; (b) SS; (c) BOD; (d) COD; (e) phenol; (f) formaldehyde. | |
 |
| | Fig. 7 Effects of operating time on single-stage membrane water flux. | |
As the filtering process went on, removal efficiency improved because a secondary membrane was formed on the primary filter surface and showed remarkable retention capacity for small molecules.42 For a single-stage membrane, the water qualities remained relatively stable after operating for 30 minutes. Removal efficiency enhanced rapidly with the decrease of membrane pore size, while water flux showed the opposite trend, as expected. For membrane 1, only limited effects were achieved for particulates, which may be attributed to the large average pore size. For membrane 2, the residual turbidity and SS were under 16.8 NTU and 39.8 mg L−1 and corresponding efficiencies reached more than 68% and 70%, respectively. For membrane 4, as the average pore size became smaller, residual turbidity was under 2.5 NTU and SS could not be detected, proving an excellent intercept for suspended solids. However, removal ratios of COD, BOD, phenol and formaldehyde had limited growth, which may be because COD in the water existed in soluble form and high efficiency was not achieved for soluble COD, BOD, phenol and formaldehyde by direct retention.
The results showed that after 300 hours the water flux through membrane 1 decreased only about 10%, implying that it was capable of maintaining lightly polluted for a long time and the cleaning interval can be much longer than 300 h. For membranes 2, 3 and 4, in the process of filtering, the water flux sharply reduced along with operation time. Water flux decreased to 20% of the initial flux after only 94 hours, 40 hours and 28 hours for membranes 2, 3 and 4, respectively, indicating that the smaller pore size caused more serious fouling, which resulted in significant reduction of water flux. Overall, single-stage membrane was unpractical due to the poor filtration performance of membrane 1 and easy to be clogged for membrane 4.
3.4.2. Multi-stage membrane filtration experiments. Based on Section 3.4.1, membranes with different pore sizes achieved good retention performance for different substances. In order to make full use of every membrane, we tested a multi-stage membrane, which was assembled by ranking membranes 1, 2, 3 and 4 on the card slots of mechanical membrane reactor. Because the water qualities are not stable until after half an hour of operation, water samples were obtained for quality (Table 4) and flux measurements (Fig. 8) after continuous operation for 60 minutes. Compared with the single-stage membranes, the water quality from the same outlet was nearly the same, indicating that a multi-stage mechanical membrane had no more advantages in the aspect of promoting retention than single-stage membrane. Flux attenuation modes of multi-stage and single-stage membrane were similar, but the cleaning cycle varied greatly. During the experiment, water flux showed no obvious attenuation for membrane 1, which was similar to the single-stage experiment. For membranes 2, 3 and 4, cleaning cycles were 150 h, 72 h and 48 h, respectively, extended by 66 h, 32 h and 20 h, respectively over the individual membrane. This was because the multi-stage membrane module can successfully intercept particles with different size ranges, making the filtering precision have a very good gradient effect. As a result, it significantly slowed the rate of membrane fouling and extended the cleaning cycle time.
Table 4 Effects of multi-stage membrane on water quality
| Membrane number |
1 |
2 |
3 |
4 |
| Turbidity (NTU) |
41.16 |
15.09 |
4.52 |
1.80 |
| SS (mg L−1) |
93.11 |
36.94 |
11.50 |
0 |
| BOD (mg L−1) |
980 |
918 |
858 |
740 |
| COD (mg L−1) |
2560 |
2475 |
2329 |
2032 |
| BOD/COD |
0.383 |
0.371 |
0.368 |
0.364 |
| Phenol (mg L−1) |
80 |
65 |
52 |
28 |
| Formaldehyde (mg L−1) |
95 |
82 |
64 |
43 |
 |
| | Fig. 8 Effects of operating time on multi-stage membrane water flux. | |
Overall, these results revealed that the O3/MgO/H2O2 could not only greatly reduce the concentration of toxic phenol and formaldehyde to an acceptable level for biological treatment processes but also improved biodegradability of the wastewater by increasing BOD/COD from 0.165 to 0.342. The combined coagulation–mechanical membrane filtration showed excellent removal for turbidity and SS as well as further COD and BOD removal. Therefore, the integration of CAOP of O3/MgO/H2O2 with coagulation–mechanical membrane filtration can effectively treat high strength phenol-formaldehyde resin wastewater.
4. Conclusions
A novel technique for phenol-formaldehyde resin wastewater pretreatment was developed and its capability was demonstrated. Turbidity was reduced from 550 to 1.8 NTU, SS was reduced from 428 to 0 mg L−1, COD was reduced from 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 to 2032 mg L−1, BOD was reduced from 2990 to 740 mg L−1, BOD/COD was increased from 0.165 to 0.364, concentration of phenol and formaldehyde were reduced to 28 mg L−1 and 43 mg L−1, respectively from the initial 4790 mg L−1 and 2660 mg L−1. The O3/MgO/H2O2 attained removal ratios of 91.8% for phenol, 85.2% for formaldehyde, 72.8% for COD and 34.9% for BOD under the following operational conditions: H2O2, MgO and O3 concentrations = 5 g L−1, 6 g L−1 and 0.25 g L−1 min−1, respectively; pH = 8; reaction time = 120 min. Formation of various types of oxidizing radicals can explain the good degradation performance. The coagulation–mechanical membrane filtration process completely removed turbidity and SS when 3 g L−1 of PAC and 30 mg L−1 CPAM were added. The four-stage mechanical membrane module can successfully intercept particles with different size ranges and significantly slowed the rate of membrane fouling. The membrane filtration effluent obtained under these experimental conditions could be post-treated in a biological system. In summary, this study indicated that the integration of an O3/MgO/H2O2 process with coagulation–mechanical membrane filtration was very efficient for the pretreatment of toxic and inhibitory phenol-formaldehyde resin wastewater.
995 to 2032 mg L−1, BOD was reduced from 2990 to 740 mg L−1, BOD/COD was increased from 0.165 to 0.364, concentration of phenol and formaldehyde were reduced to 28 mg L−1 and 43 mg L−1, respectively from the initial 4790 mg L−1 and 2660 mg L−1. The O3/MgO/H2O2 attained removal ratios of 91.8% for phenol, 85.2% for formaldehyde, 72.8% for COD and 34.9% for BOD under the following operational conditions: H2O2, MgO and O3 concentrations = 5 g L−1, 6 g L−1 and 0.25 g L−1 min−1, respectively; pH = 8; reaction time = 120 min. Formation of various types of oxidizing radicals can explain the good degradation performance. The coagulation–mechanical membrane filtration process completely removed turbidity and SS when 3 g L−1 of PAC and 30 mg L−1 CPAM were added. The four-stage mechanical membrane module can successfully intercept particles with different size ranges and significantly slowed the rate of membrane fouling. The membrane filtration effluent obtained under these experimental conditions could be post-treated in a biological system. In summary, this study indicated that the integration of an O3/MgO/H2O2 process with coagulation–mechanical membrane filtration was very efficient for the pretreatment of toxic and inhibitory phenol-formaldehyde resin wastewater.
Acknowledgements
The authors are grateful to the anonymous reviewers for their reading of the manuscript and for their suggestions and critical comments.
References
- G. Busca, S. Berardinelli, C. Resini and L. Arrighi, Technologies for the removal of phenol from fluid streams: a short review of recent developments, J. Hazard. Mater., 2008, 160, 265–288 CrossRef CAS PubMed.
- M. Ahmadi, H. Amiri and S. S. Martínez, Treatment of phenol-formaldehyde resin manufacturing wastewater by the electrocoagulation process, Desalin. Water Treat., 2012, 39, 176–181 CrossRef CAS.
- S. H. Lin and C. S. Wang, Treatment of high-strength phenolic wastewater by a new two-step method, J. Hazard. Mater., 2002, 90, 205–216 CrossRef CAS PubMed.
- C. Jarusutthirak, K. Sangsawang, S. Mattaraj and R. Jiraratananon, Treatment of Formaldehyde-Containing Wastewater Using Membrane Bioreactor, J. Environ. Eng., 2014, 138, 265–271 CrossRef.
- J. Araña, et al., Highly concentrated phenolic wastewater treatment by the Photo-Fenton reaction, mechanism study by FTIR-ATR, Chemosphere, 2001, 44, 1017–1023 CrossRef.
- X. Yang, A. Zou, J. Qiu and S. W. H. Guo, Phenol Removal from Aqueous System by Bis(2-ethylhexyl)Sulfoxide Extraction, Sep. Sci. Technol., 2014, 49, 2495–2501 CrossRef CAS.
- A. Steevensz, et al., Crude soybean hull peroxidase treatment of phenol in synthetic and real wastewater: enzyme economy enhanced by Triton X-100, Enzyme Microb. Technol., 2013, 55, 65–71 CrossRef PubMed.
- J. Cheng, S. M. Yu and P. Zuo, Horseradish peroxidase immobilized on aluminum-pillared interlayered clay for the catalytic oxidation of phenolic wastewater, Water Res., 2006, 40, 283–290 CrossRef CAS PubMed.
- G. Moussavi, The removal of formaldehyde from concentrated synthetic wastewater using O3/MgO/H2O2 process integrated with the biological treatment, J. Hazard. Mater., 2009, 171, 907–913 CrossRef CAS PubMed.
- C. Jarusutthirak, K. Sangsawang, S. Mattaraj and R. Jiraratananon, Treatment of Formaldehyde-Containing Wastewater Using Membrane Bioreactor, J. Environ. Eng., 2012, 138, 265–271 CrossRef CAS.
- P. Kajitvichyanukul, W. Wirojanagud, T. Koottatep, M. C. Lu and C. H. Liao, Degradation and detoxification of formaline wastewater by advanced oxidation processes, J. Hazard. Mater., 2006, 135, 337–343 CrossRef CAS PubMed.
- N. E. Joshi, J. E. Mccaskie and M. T. Boyle, Composition and process for treating a surface coated with a self-accelerating and replenishing non-formaldehyde immersion coating, EP, US5725640, 1998.
- J. A. O. Méndez, et al., Detoxification of waters contaminated with phenol, formaldehyde and phenol–formaldehyde mixtures using a combination of biological treatments and advanced oxidation techniques, Appl. Catal., B, 2015, 163, 63–73 CrossRef.
- C. Zhao, M. Pelaez and D. D. Dionysiou, et al., UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin, Catal. Today, 2014, 224, 70–76 CrossRef CAS.
- C. Zhao, L. E. Arroyo-Mora and A. P. Decaprio, et al., Reductive and oxidative degradation of iopamidol, iodinated X-ray contrast media, by Fe(III)-oxalate under UV and visible light treatment, Water Res., 2014, 67, 144–153 CrossRef CAS PubMed.
- B. Krishnakumar and M. Swaminathan, Influence of operational parameters on photocatalytic degradation of a genotoxic azo dye Acid Violet 7 in aqueous ZnO suspensions, Spectrochim. Acta, Part A, 2011, 81, 739–744 CrossRef CAS PubMed.
- G. Moussavi, A. Khavanin and R. Alizadeh, The integration of ozonation catalyzed with MgO nanocrystals and the biodegradation for the removal of phenol from saline wastewater, Appl. Catal., B, 2010, 97, 160–167 CrossRef CAS.
- M. Bakker, et al., Better water quality and higher energy efficiency by using model predictive flow control at water supply systems, J. Water Supply: Res. Technol.-AQUA, 2013, 62, 1–13 CrossRef.
- K. Chon, S. J. Kim, J. Moon and J. Cho, Combined coagulation-disk filtration process as a pretreatment of ultrafiltration and reverse osmosis membrane for wastewater reclamation: an autopsy study of a pilot plant, Water Res., 2012, 46, 1803–1816 CrossRef CAS PubMed.
- Y. Zhang, Preparation and Application in Wastewater Treatment of Mechanical Mechanical Module, Shandong University, 2010 Search PubMed.
- J. L. Lin, C. Huang, B. Dempsey and J. Y. Hu, Fate of hydrolyzed
Al species in humic acid coagulation, Water Res., 2014, 56, 314–324 CrossRef CAS PubMed.
- P. Jarvis, S. A. Parsons, R. Henderson, N. Nixson and B. Jefferson, The Practical Application of Fractal Dimension in Water Treatment Practice-the Impact of Polymer Dosing, Sep. Sci. Technol., 2008, 43, 1785–1797 CrossRef CAS.
- B. Han, T. Runnells, J. Zimbron and R. Wickramasinghe, Arsenic removal from drinking water by flocculation and microfiltration, Desalination, 2002, 145, 293–298 CrossRef CAS.
- G. Svehla, L. Koltai and L. Erdey, The use of 2,6-dichlorophenol-indophenol as indicator in iodometric titrations with ascorbic acid, Anal. Chim. Acta, 1963, 29(00), 442–447 CrossRef CAS.
- G. Moussavi and M. Mahmoudi, Degradation and biodegradability improvement of the reactive red 198 azo dye using catalytic ozonation with MgO nanocrystals, Chem. Eng. J., 2009, 152, 1–7 CrossRef CAS.
- American Public Health Association, American Water Works Association and Water Environment Federation, Standard Methods for the Examination of Water and Wastewater, 21st edn, 2006, pp. 130 CAS.
- S. Parra, V. Sarria, S. Malato, P. Péringer and C. Pulgarin, Photochemical versus coupled photochemical-biological flow system for the treatment of two biorecalcitrant herbicides: Metobromuron and isoproturon, Appl. Catal., B, 2000, 27, 153–168 CrossRef CAS.
- J. H. Suh and M. Mohseni, A study on the relationship between biodegradability enhancement and oxidation of 1,4-dioxane using ozone and hydrogen peroxide, Water Res., 2004, 38, 2596–2604 CrossRef CAS PubMed.
- E. Evgenidou, K. Fytianos and I. Poulios, Photocatalytic oxidation of dimethoate in aqueous solutions, J. Photochem. Photobiol., A, 2005, 175, 29–38 CrossRef CAS.
- H. M. Coleman, V. Vimonses, G. Leslie and R. Amal, Removal of contaminants of concern in water using advanced oxidation techniques, Water Sci. Technol., 2007, 55, 301–306 CrossRef CAS.
- I. Arslan, Treatability of a simulated disperse dye-bath by ferrous iron coagulation, ozonation, and ferrous iron-catalyzed ozonation, J. Hazard. Mater., 2001, 85, 229–241 CrossRef CAS PubMed.
- M. I. Pariente, et al., Heterogeneous photo-Fenton oxidation of benzoic acid in water: Effect of operating conditions, reaction by-products and coupling with biological treatment, Appl. Catal., B, 2008, 85, 24–32 CrossRef CAS.
- L. Lei, G. Li, X. Zhang and Y. Su, Catalytic oxidation of highly concentrated real industrial wastewater by integrated ozone and activated carbon, Appl. Catal., A, 2007, 327, 287–294 CrossRef CAS.
- B. D. Witte, J. Dewulf, K. Demeestere and H. V. Langenhove, Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water, J. Hazard. Mater., 2009, 161, 701–708 CrossRef PubMed.
- J. Wu and T. Wang, Ozonation of aqueous azo dye in a semi-batch reactor, Water Res., 2001, 35, 1093–1099 CrossRef CAS.
- Z. Lei, J. Ma, Z. Z. Sun and X. D. Zhai, Preliminary kinetic study on the degradation of nitrobenzene by modified ceramic honeycomb-catalytic ozonation in aqueous solution, J. Hazard. Mater., 2009, 161, 988–994 CrossRef PubMed.
- R. Li, B. Gao and H. Xin, et al., Compound bioflocculant and polyaluminum chloride in kaolin-humic acid coagulation: factors influencing coagulation performance and floc characteristics, Bioresour. Technol., 2014, 172, 8–15 CrossRef CAS PubMed.
- H. Rong, et al., Characterization of size, strength and structure of aluminum-polymer dual-coagulant flocs under different pH and hydraulic conditions, J. Hazard. Mater., 2013, 252–253, 330–337 CrossRef CAS PubMed.
- W. Xu, B. Gao, Q. Yue and W. Yan, Effect of shear force and solution pH on flocs breakage and re-growth formed by nano-Al-13 polymer, Water Res., 2009, 44, 1893–1899 CrossRef PubMed.
- K. J. Howe, M. Ashish, C. Kuang-Ping and S. S. Adham, Effect of Coagulation on the Size of MF and UF Membrane Foulants, Environ. Sci. Technol., 2006, 40, 7908–7913 CrossRef CAS PubMed.
- A. Ariffin, R. S. A. Shatat, A. R. N. Norulaini and A. K. M. Omar, Synthetic polyelectrolytes of varying charge densities but similar molar mass based on acrylamide and their applications on
palm oil mill effluent treatment, Desalination, 2005, 173, 201–208 CrossRef.
- Y. Zhang, Y. Zhao, H. Chu, B. Dong and X. Zhou, Characteristics of dynamic membrane filtration: structure, operation mechanisms, and cost analysis, Chin. Sci. Bull., 2014, 59, 247–260 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21502a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa used as polymer coagulant was purchased from Fuyuan Water Reagent Co., LTD. Formaldehyde-2,4-dinitrophenylhydrazone (FA-DNPH) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Other chemicals were supplied by Sinopharm Chemical Reagent Co., LTD. All solutions were prepared with deionized water. 1 g L−1 of 2,4-dinitrophenylhydrazine (DNPH) solution was prepared by dissolving 0.1 g DNPH into 25.0 mL HCl, followed by being diluted to 100 mL with deionized water. Formaldehyde standard solution (5 g L−1) was roughly prepared by diluting 36.0–38.0% formaldehyde solution with deionized water and then its accurate concentration was determined using the iodometric method.24 The powdered MgO nanocrystals were prepared by calcining a Mg(NO3)2 solution and the detailed production procedure is given elsewhere.25 Organic glass cylinder and copper filaments were used to prepare the mechanical membrane. Small pores with a diameter of 0.3 mm were punched uniformly on the surface of organic glass, which has a height of 20 cm and a thickness of 1 mm. On the basis of the technology in patent ZL 200810140398.3, four cylindrical mechanical membranes with different layers and diameters numbered 1, 2, 3 and 4, respectively, were successfully prepared, whose total filtration area was 344.9 cm2, 460.0 cm2, 574.9 cm2 and 689.9 cm2, respectively.
000 kDa used as polymer coagulant was purchased from Fuyuan Water Reagent Co., LTD. Formaldehyde-2,4-dinitrophenylhydrazone (FA-DNPH) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Other chemicals were supplied by Sinopharm Chemical Reagent Co., LTD. All solutions were prepared with deionized water. 1 g L−1 of 2,4-dinitrophenylhydrazine (DNPH) solution was prepared by dissolving 0.1 g DNPH into 25.0 mL HCl, followed by being diluted to 100 mL with deionized water. Formaldehyde standard solution (5 g L−1) was roughly prepared by diluting 36.0–38.0% formaldehyde solution with deionized water and then its accurate concentration was determined using the iodometric method.24 The powdered MgO nanocrystals were prepared by calcining a Mg(NO3)2 solution and the detailed production procedure is given elsewhere.25 Organic glass cylinder and copper filaments were used to prepare the mechanical membrane. Small pores with a diameter of 0.3 mm were punched uniformly on the surface of organic glass, which has a height of 20 cm and a thickness of 1 mm. On the basis of the technology in patent ZL 200810140398.3, four cylindrical mechanical membranes with different layers and diameters numbered 1, 2, 3 and 4, respectively, were successfully prepared, whose total filtration area was 344.9 cm2, 460.0 cm2, 574.9 cm2 and 689.9 cm2, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 split ratio. The column temperature was initially maintained at 100 °C for 4 min, and then temperature increased up to 250 °C at a rate of 50 °C min−1 and finally this temperature was held for 5 min. The total running time for each sample was 12 min. The FID temperature was set at 280 °C with a H2 flow of 30 mL min−1 and an air flow of 300 mL min−1.
30 split ratio. The column temperature was initially maintained at 100 °C for 4 min, and then temperature increased up to 250 °C at a rate of 50 °C min−1 and finally this temperature was held for 5 min. The total running time for each sample was 12 min. The FID temperature was set at 280 °C with a H2 flow of 30 mL min−1 and an air flow of 300 mL min−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 ± 1650 mg L−1
995 ± 1650 mg L−1


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 to 2032 mg L−1, BOD was reduced from 2990 to 740 mg L−1, BOD/COD was increased from 0.165 to 0.364, concentration of phenol and formaldehyde were reduced to 28 mg L−1 and 43 mg L−1, respectively from the initial 4790 mg L−1 and 2660 mg L−1. The O3/MgO/H2O2 attained removal ratios of 91.8% for phenol, 85.2% for formaldehyde, 72.8% for COD and 34.9% for BOD under the following operational conditions: H2O2, MgO and O3 concentrations = 5 g L−1, 6 g L−1 and 0.25 g L−1 min−1, respectively; pH = 8; reaction time = 120 min. Formation of various types of oxidizing radicals can explain the good degradation performance. The coagulation–mechanical membrane filtration process completely removed turbidity and SS when 3 g L−1 of PAC and 30 mg L−1 CPAM were added. The four-stage mechanical membrane module can successfully intercept particles with different size ranges and significantly slowed the rate of membrane fouling. The membrane filtration effluent obtained under these experimental conditions could be post-treated in a biological system. In summary, this study indicated that the integration of an O3/MgO/H2O2 process with coagulation–mechanical membrane filtration was very efficient for the pretreatment of toxic and inhibitory phenol-formaldehyde resin wastewater.
995 to 2032 mg L−1, BOD was reduced from 2990 to 740 mg L−1, BOD/COD was increased from 0.165 to 0.364, concentration of phenol and formaldehyde were reduced to 28 mg L−1 and 43 mg L−1, respectively from the initial 4790 mg L−1 and 2660 mg L−1. The O3/MgO/H2O2 attained removal ratios of 91.8% for phenol, 85.2% for formaldehyde, 72.8% for COD and 34.9% for BOD under the following operational conditions: H2O2, MgO and O3 concentrations = 5 g L−1, 6 g L−1 and 0.25 g L−1 min−1, respectively; pH = 8; reaction time = 120 min. Formation of various types of oxidizing radicals can explain the good degradation performance. The coagulation–mechanical membrane filtration process completely removed turbidity and SS when 3 g L−1 of PAC and 30 mg L−1 CPAM were added. The four-stage mechanical membrane module can successfully intercept particles with different size ranges and significantly slowed the rate of membrane fouling. The membrane filtration effluent obtained under these experimental conditions could be post-treated in a biological system. In summary, this study indicated that the integration of an O3/MgO/H2O2 process with coagulation–mechanical membrane filtration was very efficient for the pretreatment of toxic and inhibitory phenol-formaldehyde resin wastewater.





