Influence of the precursor on the porous structure and CO2 adsorption characteristics of MgO
Abstract
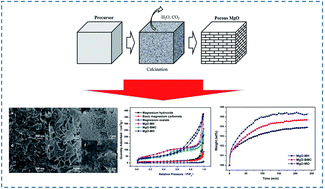
MgO is a promising candidate for CO2 capture. In general, MgO is prepared from different precursors, which have an important effect on its CO2 adsorption performance. Meanwhile, the effect of precursor on the porous structure and CO2 adsorption of MgO remains largely unknown. In this work, porous MgO was prepared from different precursors to investigate the effect of precursor source. Experimental results showed that the morphology of MgO was greatly influenced by that of the precursor and was similar to that of its precursor. Due to the emission of by-products during decomposition, MgO prepared from a precursor with a high molecular weight per single Mg atom exhibits a highly porous structure, large pore volume and pore size. The highly porous structure is favorable for the CO2 adsorption of MgO. The pores formed on the decomposition of precursor play an important role in CO2 adsorption performance, which is affected by the molecular weight per single Mg atom. Narrow pores of MgO from precursors with low molecular weights hinder CO2 diffusion and lead to a low CO2 capacity at fixed adsorption time. The results indicate that the adsorption performance of MgO could be enhanced through the regulation of precursor source and its morphology. This work provides a useful guidance for the synthesis of porous materials with high performance in CO2 capture.

 Please wait while we load your content...
Please wait while we load your content...