Hierarchical TiO2–B/anatase core/shell nanowire arrays for efficient dye-sensitized solar cells
Abstract
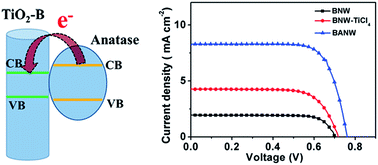
The fabrication of hierarchically structured photoanode materials is believed to be an effective way to obtain efficient dye-sensitized solar cells (DSSCs). In this paper, hierarchical TiO2–B/anatase core/shell heterojunction nanowire arrays on a titanium plate substrate are synthesized and used as novel photoanode materials for DSSCs. By using H2Ti3O7 nanowire arrays as the precursor template, anatase nanoparticle coated TiO2–B nanowire arrays are prepared via a hydrothermal reaction followed by a calcination process. Photoelectric measurements reveal that the anatase nanoparticle shell makes the pristine TiO2–B nanowire rougher for more dye adsorption and effective light scattering, which can enhance the light harvesting ability and thus the photocurrent density of the photoanode largely. Moreover, the dynamic electron transport and recombination study via electrochemical impedance spectroscopy (EIS) and intensity modulated photocurrent/photovoltage spectroscopy (IMPS/IMVS) measurements reveal that the TiO2–B/anatase composite photoanode based cell has a faster electron transport and higher electron collection efficiency than a TiO2–B based cell. As a consequence, the photoelectric conversion efficiency of the hierarchical composite photoanode was greatly enhanced to 4.88%, which is among the highest reported value for TiO2 nanowire array photoanodes grown on titanium plate substrate.


 Please wait while we load your content...
Please wait while we load your content...