Chemo- and regioselective head-to-tail heterodimerization of vinylarenes with 1,1-diphenylethene over a heterogeneous catalyst (Snβ zeolite)†
Naresh Mamedaab,
Swamy Perakaab,
Srujana Kodumurib,
Durgaiah Chevellab,
Mahender Reddy Marrib,
Hari Padmasri Aytamc and
Narender Nama*ab
aAcademy of Scientific and Innovative Research, CSIR-Indian Institute of Chemical Technology, Hyderabad, Telangana, India
bI&PC Division, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, Telangana, India. E-mail: narendern33@yahoo.co.in; nama@iict.res.in
cDepartment of Chemistry, University College for Women, Osmania University, Koti, Hyderabad 500095, Telangana, India
First published on 18th December 2015
Abstract
In the presence of Snβ zeolite, vinylarenes undergo the highly chemo- and regioselective head-to-tail heterodimerization with 1,1-diphenylethene to form the corresponding alkenes in good to excellent yields. The scope of the reaction was explored by various vinylarenes with 1,1-diphenylethene. However, the substrates with strong electron-withdrawing groups failed to react with 1,1-diphenylethene under the present catalytic conditions. Moreover, the Snβ zeolite is recyclable and can be reused without significant loss in its catalytic activity.
Introduction
The development of catalytic C–H bond activation followed by C–C bond formation reactions has been one of the most challenging and active areas of research in modern synthetic organic chemistry owing to their significant impact on both academic and industrial research.1 In particular, dimerization of alkenes is an important transformation (industrial process) for generating higher alkenes from abundant and inexpensive petrochemical feedstocks, which find extensive applications as industrial intermediates, as a source of new kinds of polymers, lubricants, detergents and many other useful chemicals.2,3 The dimerization of alkenes gives the longer chain alkenes through head-to-head (h–h), head-to-tail (h–t) and tail-to-tail (t–t) dimerization.4 Especially, the dimerization (head-to-tail (h–t) and tail-to-tail (t–t)) can be considered as the more attractive transformation since a new allylic carbon stereogenic centre is formed. In fact, several natural products and valuable pharmacologically important compounds, such as naproxen, nafenopin and dimentindene, warfarin derivative phenprocoumone5 contains this chemical structure.5 Consequently, the dimerization of alkenes has received significant attention among the scientific community.6–10Generally, 1,1-diarylethenes give the indane products in the presence of Lewis acids.11–15 However, recently, W.-M. Dai et al. developed an efficient head-to-tail heterodimerization of vinylarenes with 1,1-diarylethenes under homogenous catalytic conditions.16 But, this homogeneous catalytic system has disadvantages related to the recovery and reuse of catalyst.16 Therefore, there is a strong demand for developing methodologies for head-to-tail heterodimerization of alkenes under heterogeneous catalytic conditions in which the catalyst can be easily recoverable and reused without significant loss in their catalytic activity.
In recent years, environmental and economical considerations have raised strong interest to redesign commercially important processes to avoid the use of harmful substances and the generation of toxic waste. In this respect there is no doubt that heterogeneous catalysts can play a key role in the development of environmentally benign processes in petroleum chemistry and in the production of chemicals. Heterogeneous catalysts have many advantages over homogeneous ones such as low cost, tolerance to a wide range of temperatures and pressures, easy and inexpensive removal from the reaction mixture by simple filtration or centrifugation, easy and safe disposal, safe storage, long lifetimes, increased eco-friendliness, regenerability and reusability. Moreover, application of the inorganic solid acids, especially zeolite materials have wide spread applications both in petroleum and fine chemical industries due to their unique physical and chemical properties, such as uniform channel size, large internal surface area, unique molecular shape selectivity, strong acidity and good thermal/hydrothermal stability. In continuation of our efforts toward the development of novel and eco-friendly synthetic protocols using zeolites,17 herein we report a highly chemo- and regioselective head-to-tail heterodimerization of vinylarenes with 1,1-diphenylethene over heterogeneous catalyst (Snβ zeolite). However, to the best of our knowledge, hitherto there has been no report for the head-to-tail heterodimerization of vinylarenes with 1,1-diphenylethene using a reusable, heterogeneous catalyst.
Experimental
Reagents and materials
Zeolite β in ammonia form (NH4β) with SiO2/Al2O3 = 38 was purchased from Alfa Aesar. Vinylarenes, 1,1-diphenylethene and metal precursors were purchased from Sigma-Aldrich.Preparation of catalyst
The NH4β zeolite was calcined at 500 °C for 10 h to obtain Hβ zeolite. Metal modified zeolite Hβ was prepared by wet impregnation using the metal precursors (Fe(NO3)3·9H2O, Cu(NO3)2·3H2O, (NH4)6Mo7O24·4H2O, Ni(NO3)2·6H2O, Co(NO3)2·6H2O, (NH4)6H2W12O40·XH2O, Cr(NO3)3·9H2O and SnCl4·5H2O) with 10 weight percentage. Zeolite Hβ was added to the aqueous solution of corresponding metal salt (the ratio of weight of the zeolite to the volume of distilled water used to dissolve the metal salt was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which was then placed on a hot plate with stirring at 80 °C to remove the excess water. The prepared solid mass was dried for 12 h at 100 °C and calcined at 450 °C for 6 h in the presence of static airflow before using it for the reaction.
3), which was then placed on a hot plate with stirring at 80 °C to remove the excess water. The prepared solid mass was dried for 12 h at 100 °C and calcined at 450 °C for 6 h in the presence of static airflow before using it for the reaction.
General procedure for the head to tail heterodimerization of vinylarenes with 1,1-diphenylethene
Vinylarene (2 mmol), 1,1-diphenylethene (2.5 mmol) and 10% Snβ zeolite (100 mg) were added to 1 mL of 1,2-dichloroethane (DCE) in a 15 mL of sealed vial and the reaction mixture was allowed to stir at 100 °C. After disappearance of the vinylarene (monitored by TLC) or after an appropriate time, the reaction mixture was cooled to room temperature, diluted with DCM. The catalyst was separated by filtration and the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel using n–hexane–ethyl acetate as eluent to give desired products. All the products were identified on the basis of 1H and 13C NMR spectral data.Characterization of catalysts
All the samples were systematically characterized by different spectroscopic techniques. The XRD patterns of the samples were obtained on a Regaku miniflux X-ray diffractometer using Ni filtered CuKα radiation at 2θ = 2–80° with a scanning rate of 2° min−1 and the beam voltage and currents of 30 kV and 15 mA, respectively. FT-IR (Carry 660, Agilent Technologies) spectra were obtained in the range of 1300–1700 cm−1 with a resolution of 4 cm−1 and 64 scans. The experiments were performed in situ using a purpose-made IR cell connected to a conventional vacuum-adsorption apparatus. The sample powders were pressed into self-supporting wafers (density – 40 mg cm−3) under a pressure of 105 Pa. After that, the samples were introduced in the IR cell. Firstly, the samples were pre-treated by heating them in dynamic vacuum at a rate of 10 °C min−1 up to 400 °C and maintained at the same temperature for 1 h. After cooling down to 150 °C the spectrum was collected in the DRIFT mode. Then the samples were exposed to pyridine until surface saturation and the spectrum was recorded. The DRIFT spectra after pyridine treatment were subtracted from the spectra of the untreated catalyst to obtain the peaks only due to pyridine–acid interaction. Finally, the spectra were quantified with the Kubelka–Munk function. The temperature programmed desorption (TPD) of ammonia of Hβ and metal modified β zeolites samples were measured using an Auto Chem 2910 (Micromeritics, USA). In a typical method about 0.1 g of calcined sample was degassed at 200 °C for 3 h in helium at a flow rate of 30 mL min−1. After degassing the sample was saturated with 10% NH3 (balance helium) at 60 °C, at a flow rate of 50 mL min−1 and subsequently flushed with helium gas at 60 °C for 1 h. The TPD measurements were carried out from 60 °C to 700 °C at a ramping rate of 10 °C min−1. The amount of desorbed NH3 was calculated using GRAMS/32 software. The products were identified by NMR spectra using a Bruker VX NMR FT-300 or Varian Unity 500 spectrometers instrument in CDCl3. Chemical shifts (δ) are reported in parts per million (ppm) downfield from TMS. ESI mass spectra were obtained by using Micromass Quattro LC mass spectrometer and high-resolution mass spectra obtained by using ESI-QTOF mass spectrometry.Results and discussion
Catalyst screening
Initially, we investigated the suitable reaction conditions for head-to-tail heterodimerization of vinylarenes with 1,1-diphenylethene using styrene (1a) and 1,1-diphenylethene (2a) as a model system (Table 1). In order to choose the best catalyst, the reaction was carried out over various zeolites, MCM-41 and montmorillonite K10 at 100 °C in sealed vial for 15 h in DCE (Table 1, entries, 1–7). Among the catalysts examined, Hβ zeolite showed the higher catalytic activity and furnished the 3a in 57% yield, along with 15% yield of homodimer 4a (Table 1, entry 7). Although extensively investigated for many zeolitic structures, metal modified zeolite β has not been yet widely explored.18 It is well acknowledged that the metal modification in zeolites manipulates the total acidity, acid site strength and nature of acid sites.19 In an attempt to improve the yield of 3a, we studied the head-to-tail heterodimerization of styrene with 1,1-diphenylethene over various metal modified zeolite β catalysts under similar reaction conditions (Table 1, entries 9–15) and the results revealed that Snβ is the most effective catalyst over others (Table 1, entry 9). Influence of the both solvent and catalyst amount were also studied under similar reaction conditions over Snβ (see the ESI Tables S1 and S2†). As can be seen from above obtained results, the optimized reaction conditions to get the highest yield for this reaction are 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 mole ratio of styrene to 1,1-diphenylethene in 1,2-dichloroethane (DCE) (1 mL) at 100 °C over 10% Sn beta zeolite (Table 1, entry 9).
2.5 mole ratio of styrene to 1,1-diphenylethene in 1,2-dichloroethane (DCE) (1 mL) at 100 °C over 10% Sn beta zeolite (Table 1, entry 9).
| Entry | Catalyst | Conversion 1ab (%) | 3a yieldc (%) | 4a yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2 mmol), 2a (2.5 mmol), catalyst (100 mg), 1,2-dichloroethane (DCE) (1 mL), 100 °C, 15 h, sealed vial.b Conversion of 1a was based on GC.c Isolated yields. | ||||
| 1 | NaY | 15 | 8 | 4 |
| 2 | HY | 20 | 11 | 7 |
| 3 | HMCM-41 | 21 | 13 | 6 |
| 4 | HZSM-5 (40) | 16 | 10 | 5 |
| 5 | Montmorillonite K10 | 23 | 15 | 7 |
| 6 | H-mordenite | 26 | 16 | 8 |
| 7 | Hβ | 72 | 57 | 15 |
| 8 | Absence of catalyst | 00 | 00 | 00 |
| 9 | 10% Snβ | 99 | 90 | 9 |
| 10 | 10% Feβ | 28 | 20 | 6 |
| 11 | 10% Coβ | 25 | 18 | 7 |
| 12 | 10% Crβ | 36 | 24 | 10 |
| 13 | 10% Wβ | 43 | 30 | 11 |
| 14 | 10% Niβ | 52 | 42 | 9 |
| 15 | 10% Moβ | 76 | 63 | 11 |
Having the optimized conditions in hand, the scope and limitations of the present catalytic system was explored by reaction of various vinylarenes with 1,1-diphenylethene and the results are summarized in Table 2. The 1H NMR spectra of all products indicated that only the E isomers were formed. Alkyl substituted styrenes 1c–1f reacted smoothly to furnish the corresponding trisubstituted alkenes 3c–3f in 80–86% yields, respectively, without forming respective homodimers (Table 2, entries 3–6). In case of 4-methoxystyrene (1b), the polymerization pathway became dominant and a complex mixture of unidentified products were obtained (Table 2, entry 2). The reaction of 4-halo-styrenes 1g–1i with 1,1-diphenylethene proceeded efficiently to afford the relevant products 3g–3i in 70–76% yields (Table 2, entries 7–9), along with a substantial amounts of corresponding homodimers (16–22%). Unfortunately, the substrates 3-nitrostyrene (1j), 4-vinylbenzioc acid (1k), 2-vinylpyridine (1l) and 1-octene (1m) did not react under the present reaction conditions (Table 2, entries 10–13).
| Entry | Olefin | R | Product | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a–1m (2 mmol), 2a (2.5 mmol), 10% Snβ (100 mg), 1,2-dichloroethane (DCE) (1 mL), 100 °C, 15 h, sealed vial.b Isolated yields.c Polymerization products.d No conversion of substrate was observed. | ||||
| 1 | 1a | Ph | 3a | 90 |
| 2 | 1b | 4-MeOC6H4 | 3b | 00c |
| 3 | 1c | 3-MeC6H4 | 3c | 80 |
| 4 | 1d | 4-MeC6H4 | 3d | 86 |
| 5 | 1e | 2,4-DiMeC6H3 | 3e | 80 |
| 6 | 1f | 4-tert-Butyl C6H4 | 3f | 84 |
| 7 | 1g | 4-FC6H4 | 3g | 75 |
| 8 | 1h | 4-ClC6H4 | 3h | 76 |
| 9 | 1i | 4-BrC6H4 | 3i | 70 |
| 10d | 1j | 3-NO2C6H4 | 3j | — |
| 11d | 1k | 4-COOHC6H4 | 3k | — |
| 12d | 1l | C5H5N | 3l | — |
| 13d | 1m | n-C8H16 | 3m | — |
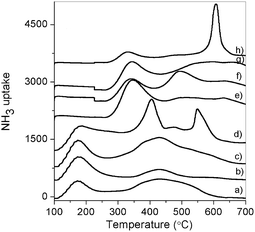
To determine the strength of acidity of the samples, temperature programmed desorption of ammonia (NH3-TPD) has been performed on the Hβ and metal modified β zeolites (Fig. 1). The total amount of acidity measured by NH3-TPD for the Hβ and metal modified zeolite β catalysts are represented in Table 3. As can be seen in Fig. 1, the presence of weak acid sites are responsible for high conversion of styrene (Snβ, Moβ and Hβ), whereas other metal modified beta zeolites have strong to medium acid sites which gave less conversion of styrene. Among the modified Hβ zeolites, Snβ was found to be most active catalyst probably due to more number of weak acidic sites present on the catalyst surface (see the ESI Table S4†).
The pyridine adsorbed Hβ and modified Hβ samples are analyzed by FT-IR spectroscopy to distinguish the Brönsted and Lewis acid sites present on the catalyst surface. The interaction of pyridine with the Brönsted and Lewis acid sites gives rise to bending vibrations at 1540 and 1450 cm−1, respectively and a vibrational band at 1490.4 cm−1 is attributed to pyridine adsorbed on both Bronsted and Lewis acid sites. The relative ratio of peak intensities corresponding to Bronsted and Lewis acid sites is measured and the results are reported in Table 3. It is evident from Table 3 that the Snβ showed a lowest B/L ratio (0.20) (than the Hβ and other metal modified beta zeolites) gave better yield of the corresponding product (3a) compared to other catalysts. Although Hβ possessed a higher B/L ratio, the TPD of NH3 results exemplified that the presence of higher number of weak acid sites compared to other metal modified Hβ zeolites could be a possible reason for the higher conversion of styrene over Hβ catalyst.
The XRD patterns of Hβ and Snβ showed similar patterns (Fig. 2(II)), except a prominent peak at 2θ = 51.7° which is attributed to SnO2 phase (ICDD# 88-0287) and this phase remain unchanged even after reuse, which indicates the stability of the catalyst.
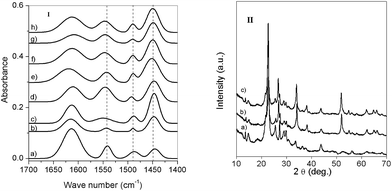 | ||
| Fig. 2 (I) Pyridine FT-IR spectra of (a) Hβ (b) Snβ (c) Moβ (d) Wβ (e) Niβ (f) Crβ (g) Feβ (h) Coβ. (II) XRD pattern of (a) Hβ (b) Snβ (c) reused Snβ. | ||
The reusability of the catalyst is one of the most significant properties for the industrial applications and environmental considerations. The catalyst (Snβ zeolite) was easily separated from the reaction mixture by simple filtration. Further, recycling of catalyst was carried out by performing the reaction of styrene with 1,1-diphenylethene under standard reaction conditions and the reused catalyst showed consistent activity even after fifth reuse (Table 4). The catalyst was highly crystalline before and after the reaction, which was confirmed by XRD (Fig. 1). There was no leaching of aluminium or silicon from zeolite (Hβ) and confirmed by elemental analysis.
A plausible reaction mechanism is illustrated in Scheme 1. It is assumed that the Lewis acidic sites of Snβ zeolite brings the olefin molecules closer by coordinating with the π electrons of the olefins. Once the olefin molecules are adsorbed and coordinated to acidic sites (both Lewis and Bronsted) of Snβ zeolite, polarization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond by Bronsted acidic site of zeolite generating an electrophilic center, which subsequently reacts with another olefin to give the corresponding dimer via intermediate (A).
C bond by Bronsted acidic site of zeolite generating an electrophilic center, which subsequently reacts with another olefin to give the corresponding dimer via intermediate (A).
Conclusions
In conclusion, we have developed a convenient Snβ zeolite catalyzed protocol for the highly chemo- and regioselective head-to-tail heterodimerization of vinylarenes with 1,1-diphenylethene under mild conditions. The scope and limitations of this process are demonstrated with various vinylarenes and 1,1-diphenylethene. However, the substrates (alkenes) having strong electron-withdrawing groups were unsuccessful with this catalytic system. Notable advantages offered by this strategy are use of non-hazardous and reusable catalyst, higher yields of the desired products, simple work-up procedure, which make this catalytic system an attractive and useful alternative to the existing method.Acknowledgements
We thank the CSIR Network project CSC-0123 for financial support. M. N., C. D. and M. M. R acknowledge the financial support from CSIR, India in the form of fellowships. P. S. and K. S. acknowledge the financial support from UGC, India in the form of fellowship.Notes and references
- (a) T. Y. Luh, M. K. Leung and K. T. Wong, Chem. Rev., 2000, 100, 3187 CrossRef CAS PubMed; (b) C. S. Chin, G. Won, D. Chong, M. Kim and H. Lee, Acc. Chem. Res., 2002, 35, 218 CrossRef CAS PubMed; (c) K. Fagnou and M. Lautens, Chem. Rev., 2003, 103, 169 CrossRef CAS PubMed; (d) D. Prajapati and M. Gohain, Tetrahedron, 2004, 60, 815 CrossRef CAS; (e) M. Shibasaki, E. M. Vogl and T. Ohshima, Adv. Synth. Catal., 2004, 346, 1533 CrossRef CAS.
- (a) J. Christoffers and R. G. Bergman, J. Am. Chem. Soc., 1996, 118, 4715 CrossRef CAS; (b) Y. Liang, G. P. A. Yap, A. L. Rheingold and K. H. Theopold, Organometallics, 1996, 15, 5284 CrossRef CAS; (c) S. Hajela and J. E. Bercaw, Organometallics, 1994, 13, 1147 CrossRef CAS; (d) A. M. Al-Jarallah, J. A. Anabtawi, M. A. B. Siddiqui, A. M. Aitani and A. W. Al-SaKdoun, Catal. Today, 1992, 14, 1 CrossRef; (e) J. Skupinska, Chem. Rev., 1991, 91, 613 CrossRef CAS; (f) W. E. Piers, P. J. Shapiro, E. E. Bunel and J. E. Bercaw, Synlett, 1990, 74 CrossRef CAS; (g) S. M. Pillai, M. Ravindranathan and S. Sivaram, Chem. Rev., 1986, 86, 353 CrossRef CAS.
- T. V. Rajanbabu, Chem. Rev., 2003, 103, 2845 CrossRef CAS PubMed.
- (a) T. Kondo, D. Takagi, H. Tsujita, Y. Ura, K. Wada and T. A. Mitsudo, Angew. Chem., Int. Ed., 2007, 46, 5958 CrossRef CAS PubMed; (b) Z. Z. Jiang and A. Sen, J. Am. Chem. Soc., 1990, 112, 9655 CrossRef CAS; (c) E. Hauptman, S. Sabo-Etienne, P. S. White, M. Brookhart, J. M. Garner, P. J. Fagan and J. C. Calabreset, J. Am. Chem. Soc., 1994, 116, 8038 CrossRef CAS; (d) J. J. Peng, J. Y. Li, H. Y. Qiu, J. X. Jiang, K. Z. Jiang, J. J. Mao and G. Q. Lai, J. Mol. Catal. A: Chem., 2006, 255, 16 CrossRef CAS.
- (a) M. Rueping, B. J. Nachtsheim and T. Scheidt, Org. Lett., 2006, 8, 3717 CrossRef CAS PubMed; (b) J. Kischel, I. Jovel, K. Mertins, A. Zapf and M. Beller, Org. Lett., 2006, 8, 19 CrossRef CAS PubMed.
- (a) T. Tsuchimoto, S. Kamiyama, R. Negoro, E. Shirakawa and Y. Kawakami, Chem. Commun., 2003, 852 RSC; (b) G. W. Kabalka, G. Dong and B. Venkataiah, Tetrahedron Lett., 2004, 45, 2775 CrossRef CAS; (c) J. Peng, J. Li, H. Qiu, J. Jiang, K. Jiang, J. Mao and G. Lai, J. Mol. Catal. A: Chem., 2006, 255, 16 CrossRef CAS; (d) C. Sui-Seng, L. F. Groux and D. Zargarian, Organometallics, 2006, 25, 571 CrossRef CAS; (e) C. Sui-Seng, A. Castonguay, Y. Chen, D. Gareau, L. F. Groux and D. Zargarian, Top. Catal., 2006, 37, 81 CrossRef CAS.
- (a) F. Dawans, Tetrahedron Lett., 1971, 12, 1943 CrossRef; (b) G. Henrici-Olivé, S. Olivé and E. Schmidt, J. Organomet. Chem., 1972, 39, 201 CrossRef; (c) C. Y. Yi, R. M. Hua and H. X. Zeng, Catal. Commun., 2008, 9, 85 CrossRef CAS.
- C. C. Wang, P. S. Lin and C. H. Cheng, Tetrahedron Lett., 2004, 45, 6203 CrossRef CAS.
- N. Galdi, C. D. Monica, A. Spinella and L. Oliva, J. Mol. Catal. A: Chem., 2006, 243, 106 CrossRef CAS.
- J. R. Cabrero-Antonino, A. Leyva-Perez and A. Corma, Adv. Synth. Catal., 2010, 352, 1571 CrossRef CAS.
- R. Wolovsky and N. Maoz, J. Org. Chem., 1973, 38, 4040 CrossRef CAS.
- M. Higashimura, K. Imamura, Y. Yokogawa and T. Sakakibara, Chem. Lett., 2004, 33, 728 CrossRef CAS.
- C. Peppe, E. S. Lang, F. Molinos de Andrade and L. Borges de Castro, Synlett, 2004, 1723 CrossRef CAS.
- H. B. Sun, B. Li, R. Hua and Y. Yin, Eur. J. Org. Chem., 2006, 4231 CrossRef CAS.
- (a) F. Ciminale, L. Lopez and G. Mele, Tetrahedron, 1994, 50, 2685 CrossRef; (b) F. Ciminale, L. Lopez, V. Paradiso and A. Nacci, Tetrahedron, 1996, 52, 1397 CrossRef; (c) F. Ciminale, L. Lopez, G. M. Farinola, S. Sportelli and A. Nacci, Eur. J. Org. Chem., 2002, 3850 CrossRef CAS.
- J. Dai, J. Wu, G. Zhao and W. M. Dai, Chem.–Eur. J., 2011, 17, 8290 CrossRef CAS PubMed.
- (a) N. Narender, K. S. K. Reddy, M. A. Kumar, C. N. Rohitha and S. J. Kulkarni, Catal. Lett., 2010, 134, 175 CrossRef CAS; (b) K. V. V. K. Mohan, N. Narender and S. J. Kulkarni, Microporous Mesoporous Mater., 2007, 106, 229 CrossRef; (c) K. V. V. K. Mohan, N. Narender and S. J. Kulkarni, Green Chem., 2006, 8, 368 RSC; (d) N. Narender, K. V. V. K. Mohan, R. V. Reddy, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, J. Mol. Catal. A: Chem., 2003, 192, 73 CrossRef CAS; (e) N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, J. Catal., 2001, 202, 430 CrossRef CAS; (f) N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, Green Chem., 2000, 2, 104 RSC; (g) M. M. Reddy, M. A. Kumar, P. Swamy, M. Naresh, K. Srujana, L. Satyanarayana, A. Venugopal and N. Narender, Green Chem., 2013, 15, 3474 RSC.
- J. N. Armor, Microporous Mesoporous Mater., 1998, 22, 451 CrossRef CAS.
- A. J. Cruz-Cabeza, D. Esquivel, C. Jimenez-Sanchidrian and F. J. Romero-Salguero, Materials, 2012, 5, 121 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and NMR spectra (1H and 13C). See DOI: 10.1039/c5ra24948a |
| This journal is © The Royal Society of Chemistry 2016 |