Rapid detection of Enterobacter cloacae by immunomagnetic separation and a colloidal gold-based immunochromatographic assay
Abstract
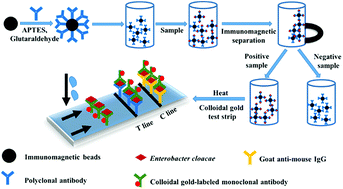
To establish the rapid detection of Enterobacter cloacae in food, immunomagnetic beads were prepared by coupling an anti-E. cloacae polyclonal antibody with magnetic beads. An immunochromatographic test strip was composited with an anti-E. cloacae monoclonal antibody marked by colloidal gold as the detection antibody, anti-E. cloacae polyclonal antibody as the test line, and donkey anti-mouse IgG secondary antibody as the control line. Immunomagnetic separation was combined with a colloidal gold-based immunochromatographic assay for the rapid detection of E. cloacae. The results showed that 102 CFU mL−1 E. cloacae could be detected using the immunochromatographic test strip after immunomagnetic separation. The sensitivity was 10 times higher than direct detection with an immunochromatographic test strip. With the immunochromatographic test strip, water samples spiked with E. cloacae yielded a negative result, whereas E. cloacae yielded a positive result with immunomagnetic separation. The proposed method enhances the rapid detection of E. cloacae in food.

 Please wait while we load your content...
Please wait while we load your content...