Application of a multifunctional magnetic mesoporous material for seafood sample clean-up prior to the determination of highly chlorinated polychlorinated biphenyls†
Abstract
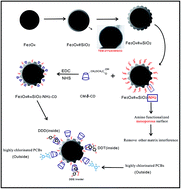
In this study, a highly effective clean-up adsorbent was developed for eliminating matrix interferences, especially main organochlorine pesticide residues during the determination of highly chlorinated polychlorinated biphenyls in seafood. The multifunctional adsorbent was prepared by grafting carboxymethyl-β-cyclodextrin on the surface of amino functionalized mesoporous nanoparticles. The amino group functionalized mesoporous SiO2 can remove most of matrix interference in samples. Moreover, carboxymethyl-β-cyclodextrin has stronger host–guest complexation with 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, 2,2-bis(p-chlorophenyl)-1,1-dichloro-ethylene, and 1,1-dichloro-2,2-bis(p-chloropheny)ethane. However, it showed weaker adsorption ability toward highly chlorinated polychlorinated biphenyls due to a steric hindrance effect. Based on this, a gas chromatography-mass spectrometry method coupled with the multifunctional adsorbent as a clean-up adsorbent for dispersive solid phase extraction was developed for the analysis of several highly chlorinated polychlorinated biphenyls in seafood samples. The results indicate that the multifunctional adsorbent as a purification material can easily and effectively remove matrix interferences in seafood samples within a short time. The recoveries for polychlorinated biphenyls were in the range of 88.4–103.2%, with relative standard deviations varying between 1.3 and 5.7%.

 Please wait while we load your content...
Please wait while we load your content...