Ion size, loading, and charge determine the mechanical properties, surface apatite, and cell growth of silver and tantalum doped calcium silicate
Abstract
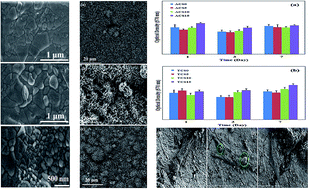
This study describes how various loadings of two ions with different size and charge, such as silver and tantalum, can affect the mechanical and biological properties of calcium silicate (CS). The incorporation of 5 wt% tantalum (Ta, charge: 5+, ion radius: 0.68 Å) in the CS lattice raised the hardness and fracture toughness values significantly. Increasing the Ta concentration to 10 wt% and 15 wt%, did not lead to further improvements. Additions of silver (Ag, charge: 1+, ion radius: 1.13 Å) to 5, 10 and 15 wt%, resulted in an increase in hardness and fracture toughness values in comparison to undoped CS; however, these values did not change significantly with different concentrations of Ag. Both Ta and Ag doped CS formed surface layers of apatite in simulated body fluid (SBF). CS was found to stimulate hFOB proliferation. The incorporation of Ta into CS did not change this and hFOB proliferation was stimulated even at higher concentrations. With increasing Ag content in the CS structure, the cell proliferation was slightly inhibited.

 Please wait while we load your content...
Please wait while we load your content...