Sensing and discrimination of cyanide and hydrogen sulfide using an 8-alkenyl-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene derivative†
E. Alvarado-Martínezc,
A. M. Costero*ab,
S. Gilab and
E. Peña-Cabrerac
aInstituto Interuniversitario de Reconocimiento Molecular y Desarrollo Tecnológico Universitat de València. Dr Moliner, 50. 26100-Burjassot, Valencia, Spain. E-mail: ana.costero@uv.es
bCIBER de Bioingeniería Biomateriales y Nanomedicina (CIBER-BBN), Spain
cDepartamento de Química, Universidad de Guanajuato. Col Noria Alta s/n, 36050, Guanajuato, Gto., Mexico
First published on 11th December 2015
Abstract
8-(2-Phenylethenyl)BODIPY has been shown to be an appropriate chromo-fluorogenic probe for cyanide in H2O. Good selectivity and LOD values below the allowed cyanide concentration in drinking water were attained. Cyanide can be discriminated from hydrogen sulphide by an oxidation process with hydrogen peroxide.
Some anions are present as contaminant species in the environment whereas others play important roles in biological systems. Among those, detection of the cyanide anion is particularly appealing as it is highly toxic1 yet it is widely used in industrial processes such as in mineral extraction, metallurgy, and in the preparation of plastics, fibers, gold, dyes and other compounds.2
Not surprisingly, several efforts have been made to address their detection, most in solution, such as electrochemical sensors,3 polymers,4 gold nanoparticles,5 CdSe quantum dots,6 and chromogenic and fluorogenic organic sensors.7 Optical sensors are especially attractive due to their reduced cost, easy detection with both high sensitivity and selectivity even by the naked eye. In addition they are suitable to sensing under biological conditions.8 In this context, 4,4′-difluoro-4-bora-3a,4a-diaza-s-indacenes, named BODIPY dyes play an outstanding role. Their synthetic versatility, allowing an easy modulation of their properties,9 makes them candidates for a wide range of applications such as chromogenic sensors, releasing drugs scaffolds, fluorescent switches, electroluminescent films, laser dyes, sensitizer for cells, fluorescent marks in biomaterials, photosensitizers in photodynamic therapy.10 Many BODIPY dyes in metallic cation sensing have been described, their applications in anion sensing are scarce.11
Recently, a general synthesis of 8-alkenylborodipyrromethenes from 8-methylthioBODIPY using a Liebeskind–Srogl cross coupling has been described.12 One of us had previously reported that the presence of an alkenyl moiety at the 8-position of the BODIPY core inhibits the fluorescence of the compound and that the transformation of the sp2 carbon atoms into sp3 through hydrogenation or Michael-like nucleophilic addition give rise to very fluorescent BODIPY derivatives.12 Taking into account this precedent we decided to study the reactivity of compound 1 with KCN in order to explore its possible use as a sensor for this dangerous anion.
Compound 1 was prepared from 8-methylthioBODIPY13 through a cross coupling Liebeskind–Srogl reaction with the corresponding boronic acid (Scheme 1).14 Compound 1 were characterized by 1H-RMN, 13C-RMN and MS (see ESI†).
The reaction of 1 with 1.5 equiv. of cyanide in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile/water for 1 h at room temperature gives rise to the expected Michel-type adduct (2) in 84% yield after chromatographic purification (Scheme 1). Compound 2 was characterized by 1H-NMR, 13C-NMR and MS (see ESI†).
1 acetonitrile/water for 1 h at room temperature gives rise to the expected Michel-type adduct (2) in 84% yield after chromatographic purification (Scheme 1). Compound 2 was characterized by 1H-NMR, 13C-NMR and MS (see ESI†).
Comparative studies of compounds 1 and 2 showed clear differences both in their UV and fluorescence spectra. Thus, whereas the UV spectra of 1 (1 × 10−5 M in CH3CN) shows two absorption bands around 410 and 510 nm the spectra of 2 presents the main absorption band at 500 nm (Fig. 1A). These absorption properties also can be naked-eye observed being the solution of 1 pink whereas the solution of 2 is colorless. On the other hand, the fluorescence emission of 1 was negligible when compared with other BODIPY-based compounds. However, compound 2 was strongly fluorescent showing an intense band at 512 nm (λex = 480 nm) (Fig. 1B).
To evaluate the utility of 1 in sensing cyanide, a titration experiment was carried out with 1 (1 × 10−5 M in CH3CN) and KCN (3 × 10−3 M in H2O, aliquots of 10 μL). The obtained results are depicted in Fig. 2. As can be seen in the figure, the addition of KCN gives rise to the progressive decrease of the bands at 410 and 510 nm with the concomitant appearance of a new band at 310 nm. The observed modifications agree with the addition of the cyanide anion to the alkenylBODIPY 1.
 | ||
| Fig. 2 Titration of 1 (1 × 10−5 M in CH3CN) and 1 (1 × 10−5 M in CH3CN) with increasing amounts of KCN (0.1–1 × 10−4 M in H2O). | ||
Surprisingly, when the emission spectra were registered, no appreciable changes in the emission intensity was observed in the titration experiment. This result was attributed to the generation, in the sensing medium, of compound I that was no fluorescent (Scheme 2). To confirm this hypothesis the solution was treated with trifluoroacetic acid to generate compound 2. As it was expected, after this treatment a strong emission band appears being the spectra identical to the fluorescence spectrum of compound 2.
To determine the selectivity of the prepared sensor, 10 equiv. of Cl−, I−, Br−, F−, HS− and SCN−, cysteine, butanethiol, sodium ethanethiolate, 2-aminoethanol and 2-(methylamino)ethanol were added. The UV spectra obtained for each anion are summarized in Fig. 3.
Cl−, I−, Br−, F− and SCN− anions did not give rise to changes in the absorption spectrum as well as cysteine, butanethiol, sodium ethanethiolate and the studied amines. However, similar changes to those observed with cyanide appeared when hydrogen sulfide was present in the solution (decreasing of the bands at 510 and 410 nm and appearance of a band around 312 nm in addition to a change of color from red to colorless). These results were expected taking into account the sensing mechanism and the fact that both, cyanide and hydrogen sulfide are similar nucleophiles. However, a clear difference between both anions arises with time as the reaction with cyanide was practically irreversible whereas the reaction with hydrogen sulfide was a reversible process that slowly regenerates the free ligand with de corresponding recovering of the pink color (Fig. 4). Due to this different behavior both anions CN− and SH− can be separately identified after 12 hours.
 | ||
| Fig. 4 Absorption spectra of 1 (1 × 10−5 M in CH3CN, black line), 1 + 5 equiv., of HS− (dotted line) and 1 + 5 equiv. HS− after 24 hours (grey line). | ||
In order to allow a fast differentiation between cyanide and hydrogen sulfide, the regeneration reaction from the sulfide adduct was induced by using hydrogen peroxide able to remove the anion HS− from the solution through an oxidation process. After this treatment both cyanide and hydrogen sulfide could be quickly differentiated as can be seen in Fig. 5.
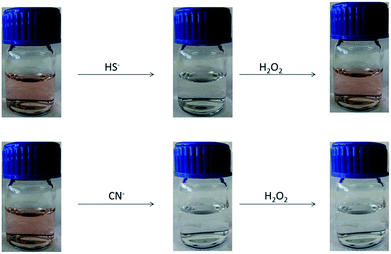 | ||
| Fig. 5 Different visual behavior of 1 (1 × 10−5 M in CH3CN) in front of 10 equiv. of CN− and HS− (both 10−4 M in H2O). Subsequent addition of 10 μL of H2O2 (6v). | ||
Sensor 1 also can be used as a fluorescent sensor for cyanide. Thus, addition of increasing amounts of cyanide followed by treatment with trifluoracetic acid give rise to a continuous enhancement of the fluorescence emission (Fig. 6). Similar results even though of lower intensity were observed immediately after the addition of hydrogenosulfide (see ESI, Fig. SI-4†).
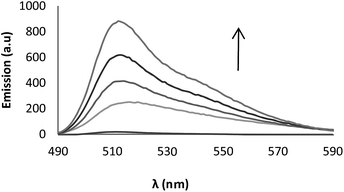 | ||
| Fig. 6 Titration of 1 (1 × 10−5 M in CH3CN) and 1 (1 × 10−5 M in CH3CN) with KCN (0.1–2.5 × 10−5 M) and subsequent treatment with trifluoracetic acid to protonate intermediate I (λex = 480 nm). | ||
The titration experiment allowed us to determine the limit of detection (LOD) using the linear part of the graphic of absorbance or fluorescence in front of concentration of the anion (see ESI, Fig. SI-5 and SI-7† for cyanide and SI-6† for hydrogenosulfide. The smaller changes observed in fluorescence after addition of hydrogenosulfide do not make this technique convenient to determine the corresponding LOD).
From these graphic, a LOD of 20 ppb and 8.3 ppb were determined for cyanide from absorbance and fluorescence data respectively. These values are lower that the permitted level of cyanide in drinking water according to the EPA (800 ppb).15 LOD for hydrogenosulfide was 78 ppb for UV data.
Conclusions
In summary, the 8-alkenylBODIPY 1 is an appropriate chromo-fluorogenic probe for cyanide and hydrogenosulfide dissolved in H2O. The LOD attained by the probe 1 are 20 ppb and 8.3 ppb for cyanide using absorbance and fluorescence respectively and 78 ppb for hydrogenosulfide using absorbance measurements. These values are lower that the allowed cyanide concentration in drinking water. In the case of cyanide, the mechanism has been confirmed by the synthesis of compound 2. Selectivity in front of a large number of nucleophiles has been established. Finally, discrimination between cyanide and hydrogen sulphide can be achieved by carrying out an oxidation process with hydrogen peroxide.Acknowledgements
We thank Spanish Government, European FEDER funds (MAT2012-38429-C04-02) and Generalitat Valenciana (PROMETEOII/2014/047) for support. Universidad de Valencia (SCSIE) is acknowledged for all the equipment employed. We wish to thank CONACyT for financial support (grants 129572 and 123732) and for graduate scholarship. Cuantico de Mexico (http://www.cuantico.mx) is gratefully acknowledged for kind donation of 8-methylthioBODIPY.Notes and references
- K. W. Kulig, Cyanide Toxicity, U. S. Department of Health and Human Services, Atlanta, GA, 1991 Search PubMed.
- (a) P. A. Patnaik, A Comprehensive Guide to the Hazardous properties of Chemical Substances, van Nostrand Reinhold, New York, 1992, p. 229 Search PubMed; (b) C. Young, L. Tidwel and C. Anderson, Cyanide: Social, Industrial and Economic Aspects: Minerals, Metals, and Materials Society, Warrendale, PA, 2001, p. 35 Search PubMed.
- A. E. Lindsay and D. O'Hare, Anal. Chim. Acta, 2006, 558, 158 CrossRef CAS.
- S. Vallejos, P. Estévez, F. C. García, F. Serna, J. L. de la Peña and J. M. García, Chem. Commun., 2010, 46, 7951 RSC.
- H. J. Kim, K. C. Ko, J. H. Lee, J. Y. Lee and J. S. Kim, Chem. Commun., 2011, 47, 2886 RSC.
- A. Touceda-Varela, E. I. Stevenson, J. A. Galve-Gasión, D. T. F. Dryden and J. C. Mareque-Rivas, Chem. Commun., 2008, 1998 RSC.
- (a) Z. Xu, X. Chem, H. N. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 127 RSC; (b) S. Bhardwaj and A. K. Singh, J. Hazard. Mater., 2015, 296, 54 CrossRef CAS PubMed; (c) F. Wang, L. Wang, X. Chen and J. Yoon, Chem. Soc. Rev., 2014, 43, 4312 RSC; (d) Y.-W. Liu, M.-X. Kao and A.-T. Wu, Sens. Actuators, B, 2015, 208, 429 CrossRef CAS; (e) R. Gotor, A. M. Costero, S. Gil, M. Parra, R. Martínez-Máñez, F. Sancenón and P. Gaviña, Chem. Commun., 2013, 59, 5669 RSC; (f) M. Dagilienė, V. Martynaitis, V. Kriščiūnienė, S. Krikštolaitytė and A. Šačkus, ChemistryOpen, 2015, 4, 363 CrossRef PubMed.
- (a) M. Sameiro and T. Gonçalves, Chem. Rev., 2009, 109, 190 CrossRef; (b) W.-H. Ding, D. Wang, X.-J. Zheng, W.-J. Ding, J.-Q. Zheng, W.-H. Mu, W. Cao and L.-P. Jin, Sens. Actuators, B, 2015, 209, 359 CrossRef CAS.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed; (b) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC; (c) N. A. M. Pereira and T. M. V. D. P. E. Melo, Org. Prep. Proced. Int., 2014, 46, 183 CrossRef CAS.
- (a) M. Takahashi, A. Kawamura, N. Kato, T. Nishi, I. Hamachi and J. Ohkanda, Angew. Chem., 2012, 124, 524 (Angew. Chem., Int. Ed., 2012, 51, 509) CrossRef; (b) D. Ye, G. Liang, M. L. Ma and J. Rao, Angew. Chem., 2011, 123, 3294 (Angew. Chem., Int. Ed., 2011, 50, 3236) CrossRef; (c) T. Bura, P. Retailleau and R. Ziessel, Angew. Chem., 2010, 122, 6809 (Angew. Chem., Int. Ed., 2010, 49, 6659) CrossRef; (d) J.-Y. Liu, E. A. Ermilov, B. Rçder and D. K. P. Ng, Chem. Commun., 2009, 1517 RSC; (e) F. Camerel, L. Bonardi, M. Schmutz and R. Ziessel, J. Am. Chem. Soc., 2006, 128, 4548 CrossRef CAS; (f) Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. Tatar Yildirim, A. L. Dogan, D. Guc and E. U. Akkaya, Angew. Chem., 2011, 123, 12143 CrossRef; (g) S. G. Awuah, J. Polreis, V. Biradar and Y. You, Org. Lett., 2011, 13, 3884 CrossRef CAS; (h) Z. Ahao, B. Chen, Z. Chang, L. Aparicio-Ixta, H. Nie, C. C. Goh, L. G. Ng, A. Qin, G. Ramos-Ortiz, B. Liu and B. Z. Tang, Part. Part. Syst. Charact., 2014, 11, 481 Search PubMed; (i) T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953 RSC.
- (a) X. Qi, E. J. Jun, L. Xu, S. J. Kim, J. S. J. Hong, Y. J. Yoon and J. Yoon, J. Org. Chem., 2006, 71, 2881 CrossRef CAS PubMed; (b) T. Cheng, Y. Xu, S. Zhang, W. Zhu, X. Qian and L. Duan, J. Am. Chem. Soc., 2008, 130, 16160 CrossRef CAS; (c) S. C. Dodani, Q. He and C. J. Chang, J. Am. Chem. Soc., 2009, 131, 18020 CrossRef CAS; (d) D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194 CrossRef CAS; (e) L. Zeng, E. W. Miller, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 10 CrossRef CAS; (f) M. Yuan, Y. Li, J. Li, C. Li, X. Liu, J. Xu, H. Liu, S. Wang and D. Zhu, Org. Lett., 2007, 9, 2313 CrossRef CAS PubMed; (g) S. Atilgan, T. Ozdemir and E. U. Akkaya, Org. Lett., 2008, 10, 4065 CrossRef CAS; (h) R. Guliyev, S. Ozturk, E. Sahin and E. U. Akkaya, Org. Lett., 2012, 14, 1528 CrossRef CAS; (i) R. Misra, T. Jadhav, B. Dhokale and S. M. Mobin, Dalton Trans., 2015, 44, 16052 RSC; (j) J. Zhang, S. Zhu, L. Valenzano, F.-T. Luo and H. Liu, RSC Adv., 2013, 3, 68 RSC.
- I. J. Arroyo, R. Hub, B. Z. Tang, F. I. López and E. P. Cabrera, Tetrahedron, 2011, 67, 7244 CrossRef CAS.
- 8-MethylthioBODIPY was donated by Cuantico de Mexico (http://www.cuantico.mx).
- L. S. Liebeskind and J. Srogl, J. Am. Chem. Soc., 2000, 122, 11260 CrossRef CAS.
- EPA safe drinking water http://water.epa.gov/drink/contaminants/.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23307k |
| This journal is © The Royal Society of Chemistry 2016 |




