Structural evolution of CeO2-doped alkali boroaluminosilicate glass and the correlation with physical properties based on a revised structural parameter analysis
Zhenlin Wang*ab and
Laifei Chenga
aScience and Technology on Thermostructural Composites Materials Laboratory, Northwestern Polytechnical University, Xi'an 710072, China. E-mail: wzl@cqut.edu.cn
bCollege of Materials Science and Engineering, Chongqing University of Technology, Chongqing 400054, China
First published on 23rd December 2015
Abstract
Alkali boroaluminosilicate glasses with composition of 56SiO2–20B2O3–3Al2O3–8Na2O–8K2O–5BaO containing 0–9 mol% cerium oxide (CeO2) were synthesized at 1500 °C using a conventional melt-quench method. Structural evolution of the as-prepared glasses was studied using infrared and Raman spectroscopy and the glasses' physical properties were characterized. The structural parameter based on the Yun, Dell and Bray model was revised by factoring multiple oxides, including CeO2, into the computation of the ratio of alkali oxide to boron trioxide, and was used to describe the glasses' structural states divided into three categories with 3–4% as the critical content that marks the onset of drastic variations in glass structure and properties. The addition of CeO2 below 3% are absorbed by reedmergnerite- and danburite-like groups producing one non-bridging oxygen (NBO) on the silica tetrahedrals and disconnecting tetrahedral borate [BO4] from tetrahedral silicate [SiO4]. Addition of CeO2 above 3% allows the additional oxygen to combine with reedmergnerite- and danburite-like units producing two NBOs on [SiO4] and also one or two NBOs on trigonal planar borate [BO3] at the expense of [BO4]. Further addition of CeO2 beyond 5% will cause the extra NBOs to gradually combine with the disconnected boron triangles to form boron tetrahedrals in addition to continuous depolymerization of the Si–O network. Network depolymerization, enhanced linkage by charge compensation and improved compactness because of close packing are proposed as the cause of diverse variation trends in physical properties in the presence of CeO2 below and above 3–4%, respectively. The revised structural parameter analysis is explainable by the correlation between the observed structural evolution and the physical properties, and thus, can be a useful reference for constituents' regulation of CeO2-doped borosilicate radiation resistant glasses.
1. Introduction
Borosilicate glasses have been used extensively for industrial applications including heat resistant wares, glazes, photoelectron devices, immobilization of irradiative waste and so on because of their low melting temperatures, minimal thermal expansion coefficients, devitrification resistance, promising optical and mechanical properties, outstanding thermal stability and high chemical durability.1 In particular, borosilicate possesses a superior dissolving capacity for rare-earth (RE) in the silicate system, and thus favors developing RE-containing optical glasses.2,3Because borosilicate glasses have always been preferentially used for radioactive waste immobilization,4,5 aerospace facility components, for example, solar cell covers6 are very likely under a harsh irradiative environment that raises concerns about their radiological safety and stability; a sufficiently guaranteed irradiation resistance should be a vital prerequisite for their proper use. A considerable number of recent studies on borosilicate glasses mixed with various modifier oxides, particularly some transition metal ions, have been enthusiastically pursued with a view to improving their radiation resistance.7–9 Cerium oxide (CeO2) has previously been found to make glasses resistant to radiation damage,10,11 and the mechanism for cerium to function as a radiation stabilizer is attributed to its polyvalent states namely Ce4+ and Ce3+ in glass via ceric–cerous redox equilibrium. They give irradiation shielding to glasses because Ce3+ captures holes and Ce4+ traps electrons yielded by irradiation to avoid their recombination, and thus to restrain the formation of color centers in the host glass.12,13 Borosilicate glasses doped with CeO2 exhibit improved irradiation resistance14,15 and potentially have wide use where there is ambient irradiation such as aerospace, nuclear facilities, photovoltaic devices as well as medical and military fields. Addition of CeO2 into borosilicate glass causes variations in physical properties which have been proved to be closely connected with CeO2 induced structural changes.16
Structural studies on borosilicate glass modified by alkali metal oxides have provided some insights into its structure using well established models. The initial model proposed by Yun, Dell and Bray17–19 uses two composition related structural parameters to describe structural features of the simple silicon dioxide–sodium oxide–boron trioxide (SiO2–Na2O–B2O3) ternary system. This provides a valuable approach to determine the possible structural evolution upon addition of alkali metal oxide although it had received much criticism and had consistently been revised and developed from then on.20,21 Structure studies relating to borosilicate glass modified by metal oxides such as cadmium oxide,7 Bi2O3,8 SrO,22 PbO9 for radiation resistance purposes have been widely conducted, whereas CeO2 is sometimes involved just as a non-radioactive surrogate to simulate the presence of minor actinides in nuclear glasses.23,24 Even so, understanding the structure-property correlations of cerium-containing borosilicate glass especially for complex multi-component system remains limited, for example, recent reviews on borate and borosilicate glasses have comprehensively summarized the effects of different additives but unfortunately without mentioning cerium.25,26
More importantly, the role cerium plays in glass structure so far remains debatable. Studies of the effect of CeO2 on aluminosilicate glass,27 borosilicate glass,16 lead silicate glass,28 borate glass29,30 and phosphate glass31,32 generally accepted that the role of cerium was to act as a network modifier. Nevertheless, some of the investigations on the structure of borate,33,34 and phosphate glasses35 containing CeO2, instead suggested that in these glasses cerium ions play a dual role, as both a network former and a network modifier depending on its content. In contrast, the structure of alkali boroaluminosilicate glasses is more complex because of the presence of multiple types of oxides, and doping CeO2 in such a system is expected to arouse intricate structural speciation leading to macroscopic property variation. Furthermore, additional oxygen introduced by CeO2 may raise the problems on how to reassess the structural parameters and how to interpret the related structural evolution based on the Yun, Dell and Bray model. In this work, the aim was to extend the applicability of this model by factoring multiple oxides into the computation of the R value to illustrate the structural changes of boroaluminosilicate glass induced by CeO2. The correlations between structural evolution and variations in physical properties were tentatively elaborated based on structure parameter analysis combined with semi-quantitative spectroscopic characterization with the aim of guiding the tailoring of the performances of CeO2 doped boroaluminosilicate glasses used in ambient irradiation.
2. Experimental
2.1 Glass preparation
The base glass with a fixed composition 56SiO2–20B2O3–3Al2O3–8Na2O–8K2O–5BaO (molar ratio) was synthesized using a conventional melt-quench method using analytically pure reagents SiO2, aluminium hydroxide, B2O3, sodium carbonate, potassium carbonate, barium carbonate, and CeO2 as starting materials. Similarly CeO2 doped glasses were fabricated by just adding a certain percentage of CeO2 powder (mol%) over the base glass (keeping the ratio of base components constant). Prior to melting, batches of mixtures had been ball milled for 2 h. The batched mixture was heated to 1500 °C within 75 min and kept melting for 5 h for complete fining in an open platinum crucible under an air atmosphere, and afterwards the melt was poured onto a preheated steel plate and was pressed to form a disk and was then annealed in a muffle furnace at 550 °C for 1 h to eliminate the internal stress. The samples were ultrasonically cleaned in acetone and then in anhydrous ethanol successively. Glass samples were referred to by their doped molar percentage of CeO2 relative to the base glass (in moles), i.e., 0–9% with a step of 1 mol%, respectively.2.2 Spectroscopic analysis
Infrared (IR) spectra measurements of the glasses from 400 to 4000 cm−1 were performed at room temperature with resolution of 2 cm−1 using a Fourier transform infrared spectrometer (FTIR, Nicolet iS10) using the standard potassium bromide (KBr) pellet method. The recorded spectrum of the pulverized sample pellet was subtracted from that of the matrix KBr. Raman spectra in the range of 200–2000 cm−1 were recorded at room temperature on a LabRAM HR800 micro-spectrometer (Horiba Jobin Yvon) using the 514.5 nm line of an Ar+ laser with the measured power of 5 mW, and the resolution of the system was less than 1 cm−1.2.3 Measurement of physical properties
Density was measured in accordance with the Archimedes principle using xylene as an immersion liquid at room temperature. Measurements were repeated three times and the densities (d) were determined by applying eqn (1) and then averaged. In eqn (1) ρb is the density of the buoyant substance, Wa and Wb are the sample weights in air and the buoyant substance, respectively.| d = ρbWa/(Wa − Wb) | (1) |
The molar volume (Vm) was calculated using eqn (2):
 | (2) |
Measurements of mechanical properties were carried out using an indentation test with a Nanotest system (Micro Materials Limited).36,37 Hardness (H) and elastic modulus (E) of the glass were first determined using a low-load indentation test setting the maximum load to 500 mN and the loading/unloading rate to 5 mN s−1, and the results were calculated using the software included with the instrument, using eqn (3) and (4) which were based on the Oliver and Pharr method,38 where P is the maximum load, A is the projected contact area, and dP/dh represents the slope of the initial portion of the unloading curve. Subsequently a high-load indentation test was conducted setting the load to 5 N and the loading/unloading rate to 50 mN s−1. The flawing impression was captured in situ to determine the fracture toughness (KIC) using eqn (5),39 where a is the length measured from the center of the indentation to the crack tip, l is the radial length of the crack and c is the length measured from the center of contact to the end of the corner radial crack.
 | (3) |
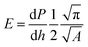 | (4) |
 | (5) |
Spectrophotometric analysis using a T6 UV-visible (UV-Vis) spectrophotometer (Persee, Beijing, China) was carried out to measure the transmittance of the prepared glass as a function of wavelength from 190 to 1100 nm with a resolution of 1 nm and then the absorption coefficient was obtained according to the Beer–Lambert law.
3. Results
3.1 Glass structure
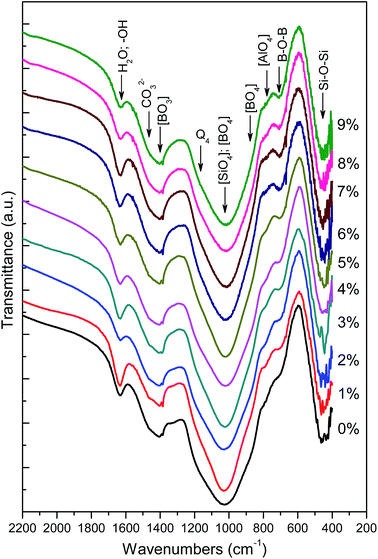
Furthermore, the bands occurred at 1400 cm−1 because of the asymmetric stretching relaxation of the B–O bond of trigonal borate ([BO3]) units25,44,45 and a shoulder at 880 cm−1 is associated with B–O stretching in [BO4].46,47 With the increase of CeO2 below 5%, the [BO3] tend to intensify and concurrently the [BO4] gradually reduces, which is likely to be related to the dominant conversion of [BO4] to [BO3] in the presence of additional oxygen upon doping with CeO2. Progressively increasing CeO2 over 5% appears to stop this trend and this implies suppressed conversion of [BO4] to [BO3]. A weak band at 781 cm−1 corresponds to the bending of the Al–O bond in the tetrahedral aluminate ([AlO4]),48,49 which appears to be unaffected by CeO2 addition because aluminum has already existed in tetrahedral form in preference to boron because of the more alkaline oxide than aluminium oxide (Al2O3) in the base glass. In addition, there is a band located at about 710 cm−1 arising from the bending of B–O–B25,50 and another one at 450 cm−1 because of the bending of the Si–O–Si bond,43,51 however this gives less clear information about the structural variation.
Assignment of band 635 cm−1 remains controversial53 because it can be attributed to either the breathing mode of B–O–Si(B) bonds from danburite- and reedmergnerite-like rings consisting of [SiO4] and [BO4] tetrahedrals52,53 in a mixed borosilicate phase or the bending of ring-type metaborate groups mainly composed of boron triangles.1,26 Considering this band weakens with rising CeO2 content, it tends to be attributed to the former because the weakening band at 635 cm−1 should signify intensifying breakage of B–O–Si(B) bonds in borosilicate rings, which are built of silicon and boron tetrahedrals rather than boron triangles. Otherwise, its trend in variation may conflict with those of other bands as well as the previously mentioned IR results.
The bands across the range 800–1200 cm−1 associated with the stretching mode of terminal groups of the silicon–oxygen tetrahedrons Qn (n is the number of bridging oxygen atoms) are of much more significance for the analysis of structural changes,55 and thus are specifically elaborated in Fig. 3. As this broad envelope comprises component peaks situated amid 780–890 cm−1, 900–940 cm−1, 950–1020 cm−1, 1060–1130 cm−1 and 1150–1200 cm−1 assigned to the Si–O stretching of Qn with n being 0, 1, 2, 3 and 4, respectively,52,54–56 peak deconvolution using several Gaussians are employed to fit multipeaks at these bands. In this study, satisfactory fitting results with negligible errors in peak center and area were acquired with accuracy which conformed to the appropriate fitting parameters (reduced chi-square < 10−5, coefficient of determination R2 > 0.999). The total area of the fitting peaks was used to normalize the individual deconvolved peak generating the proportional area of every constituent peak that represents the relative intensity of symmetric stretching of Qn. The band intensity is assumed to be proportional to the structural species' area fraction that can be used to roughly evaluate the relative Qn fraction in the glass structure.
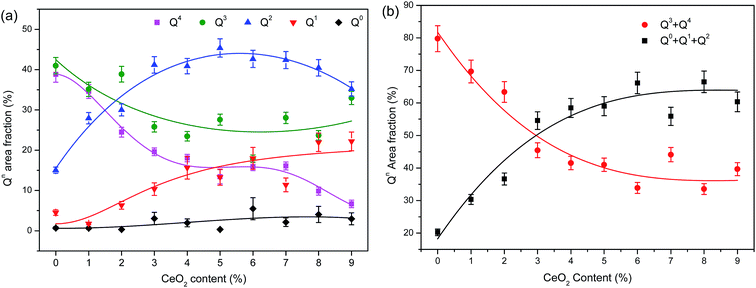
Area fraction subtotals of various Qn species as a function of CeO2 content are displayed in Fig. 4(a) where the fitted curves are shown as a visual guide. As revealed in Fig. 4(a), the base glass builds its structure mainly using higher level tetrahedra (Q4 and Q3) whose fractions decrease remarkably while the Q1 and Q2 fractions grow nonlinearly with CeO2 additions below 5%, and up to and after 3% within this range, different rates of change are exhibited. Q1 and Q2 fractions growing nonlinearly upon CeO2 additions below 5%, and up to and after 3% within this range different changing pace are exhibited. Addition of CeO2 exceeding 5% allows a gradual drop of Q2 but a rise of Q3, which roughly conforms to the transformation relationship: 2Q2 → Q3 + Q1.57 To avoid the possible uncertainty on assigning a few wavering bands located between different Qn species, the similar items specified as higher levelled Qn (Q3 and Q4) and lower levelled ones (Q0, Q1 and Q2) are merged separately into two classes. Fig. 4(b) reveals the gradual drop of the summed area fraction of Q3 and Q4 compared to the rising total of Q0, Q1 and Q2. There is an evident turning point close to a level of 3% of CeO2 signifying the nonlinear evolution of silicate structural units upon addition of CeO2. For the glasses investigated, this could be attributed to the deepening depolymerization and reconfiguration of the network starting at about 3–4% CeO2 that can be viewed as a critical content of structural evolution.
3.2 Physical properties
| kE = B(E − Eopt)n | (6) |
 | ||
| Fig. 8 (a) A demonstration of the plots for the computation of the optical band gap using UV-Vis spectra; (b) Eopt variation as a function of CeO2 content (the dashed line is shown as a guide). | ||
As exhibited in Fig. 8(b), initially Eopt decreases gradually and then declines steeply with increasing CeO2 content below 5%. As stated in the previous section, the doped CeO2 in glass acting as a network modifier creates additional NBOs by breaking up the Si–O network. However, cerium ions with a high atomic number will greatly augment the electron population density distribution in the network. The weakened covalence bonds stemming from the depolymerized network in combination with the increased electron density distribution will contribute to the shrinkage of Eopt. Furthermore, further increasing the CeO2 content beyond 3% seemingly intensifies most the two influences that allow a drastic drop of Eopt. Nevertheless, decrease in Eopt apparently slows down with CeO2 doping ≥5%, indicating that the newly introduced NBOs are reused for bridging the structural groups such as conversion of some [BO3] to [BO4] as revealed by IR and Raman spectrometry analysis.
4. Discussion
Composition of the glass studied in this research comprises multiple network formers, namely, Si, B and Al, and it is very likely that these formers allow the mixing of the borate and aluminosilicate network because of the appropriate constituent relationship. According to the model proposed by Yun, Dell and Bray, structural parameters R = M2O/B2O3 and K = SiO2/B2O3 (in molar ratio) govern the possible structural speciation upon addition of alkali oxides (M2O) in a simple ternary system, for example, Na2O–B2O3–SiO2 glasses, as follows.1,19 For R < 0.5, the borate present in a triangular structure together with those which have a tetrahedral structure, because of the conversion of [BO3/2] to [BO4/2]−, are compensated by M+ for electric neutrality. For 0.5 < R < Rmax (0.5 + K/16), formation of reedmergnerite- and danburite-like structural units occurs. Whereas for Rmax < R < Rd1 (0.5 + K/4), additional modifiers start to cause depolymerization of the network yielding NBOs in silica tetrahedrons. For Rd1 < R < Rd3 (2 + K), the additional modifier is assumed to produce NBOs on a trigonal borate at the expense of tetrahedral units as well as two NBOs on [SiO4].Analogously, CeO2 as a modifier is expected to yield additional oxygens leading to either depolymerization of the silicate network or variation in the coordination number of formers with cerium ions acting for charge balance.27 Here, multiple oxides including CeO2 are factored into the computation of the R value. In view of the polyvalence of cerium, the average additional oxygen of one mole of cerium ions can be equivalent to x mole alkali oxide expressed as a function of [Ce3+]/[Ce4+] given in eqn (7), where items in square brackets represent the ingredient's content. Here [Ce3+]/[Ce4+] strongly relies on the CeO2 concentration as well as the redox conditions of glass synthesis. Apparently, the value of x ranges from 1.5 to 2 irrespective of the scale of [Ce3+]/[Ce4+]. Correspondingly, the structural parameter R can be rewritten as eqn (8). In essence, the amended R represents the relative amount of modifiers' content for charge compensation.
 | (7) |
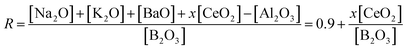 | (8) |
Calculation of the R of the base glass without CeO2 gives the value of 0.9, which falls in the range between Rmax (0.675) and Rd1 (1.2), meaning that there is the presence of mixed of 3- and 4-fold coordinated boron and silicate tetrahedral with varying NBOs (Qn) configured partly in reedmergnerite- and danburite-like groups according to the above model. Additional alkalis (K+, Na+, Ba2+) in excess of Rmax are absorbed by reedmergnerite- or danburite-like groups producing NBOs on silicate in the base glass. An Si–O–B mixed network constructed of reedmergnerite- and danburite-like groups is verified by characteristic band in the Raman spectrum at 630 cm−1 in Fig. 2. The typical three-dimensional structural models of these two groups are illustrated in Fig. 9(a) for reedmergnerite and Fig. 9(b) for danburite, which shows that the significant difference between them is that double boron tetrahedrals are included in the danburite-like group whereas there is only one connecting to four surrounding silica tetrahedrals in the reedmergnerite-like group.
 | ||
| Fig. 9 Structural modeling graphics of (a) reedmergnerite-like unit and (b) danburite-like unit assumed to be present in the base glass (red: O, blue: Si, yellow: B, grey: alkali metal). | ||
For CeO2-doped glasses, CeO2 as a modifier behaving similarly to additional alkali or alkali earth metallic oxides will readily be absorbed to a vitreous structure causing NBOs in silica network and degrading the connective dimensionality of boron in excess of the rated doping amount. This is shown by the Raman and IR spectra in the previous sections that revealed the progressive rupture of the silicate network as well as part conversion of borate tetrahedrons to triangles upon CeO2 addition. The incremental modifier above R of base glass because of the CeO2 addition is dependent on the term on the far right of eqn (8). Given that x equals 1.5–2 and [B2O3] is 20 mol%, R can be computed, using eqn (8), to be 1.2 in the presence of 3% CeO2, a value close to Rd1. That is to say, 3% is seems to be the theoretical critical CeO2 concentration that just divides the structural parameter R into two distinct categories, i.e., Rmax < R < Rd1 and R > Rd1 which correspond to the two respective structural states for the studied glass composition containing CeO2 below 5%. Furthermore, by raising the CeO2 concentration it may give rise to R until approaching a new boundary value Rd3 (4.8) roughly corresponding to the glass containing 5% CeO2. Further increasing the CeO2 content over 5% allows for R > Rd3 that has been rarely considered by the Yun, Dell and Bray model.
For the first structural state with CeO2 <3% (Rmax < R < Rd1), the added CeO2 in trivalent and tetravalent states are absorbed by reedmergnerite- and danburite-like groups producing one NBO on the silica tetrahedrals and disconnecting [BO4] from [SiO4] at one corner as shown in Fig. 10(a) and (b). In this case, the Qn units gradually degrade to lower level ones and boron triangles continuously yield upon adding CeO2. However, with increasing CeO2 from 3% to 5% in a stepwise manner, the glass structure runs into the second state (Rd1 < R < Rd3), and in return allows the additional oxygen to combine with reedmergnerite- and danburite-like units progressively rupturing the Si(B)–O skeletal network with two NBOs on [SiO4] and also one or two NBOs on [BO3] at expense of [BO4] as illustrated in Fig. 10(a′) and (b′), respectively. These steps continue until mostly [BO3] groups with two NBOs form when increasing the CeO2 content. When further adding CeO2 in excess of 5% with R > Rd3, the extra NBOs yielded will gradually combine with the disconnected boron triangles from the [SiO4] network forming boron tetrahedrals in addition to continuous depolymerization of the Si–O network. In this case, the B–O network achieved enhancement to some extent despite loosening the Si–O skeletal network, and consequently the network can maintain relative stability. This seems to explain the structural features arrived at in the forgoing sections. Thus, a level of 3–4% should be viewed as the approximate critical content that marks the onset of most of the extreme variations in properties as well.
 | ||
| Fig. 10 Assumed structural variations upon adding CeO2 (a) reedmergnerite-like, CeO2 < 3%, (a′) reedmergnerite-like, CeO2 > 3%, (b) danburite-like, CeO2 < 3%, (b′) danburite-like, CeO2 > 3%. | ||
Under these circumstances, cerium ions can occupy network interstitial sites with Ce3+ associated with three neighboring Q3 tetrahedrals and Ce4+ with four for charge compensation. Several possible configuration modes but not limited to these by virtue of electrostatic interaction are depicted in Fig. 10 and correspond to diverse structural states. Apparently, the scope of such functionality extends drastically when double NBOs are produced on silicon tetrahedrals in case of CeO2 levels beyond 3%. It is supposed that cerium ions are much more tightly bonded into the skeletal network providing stronger network linkages and immobilizing the relative displacement of loosened chains. This structural modification enhances to some extent the network interconnectivity despite local scission of the Si(B)–O bond. This postulation is essentially in agreement, but from a different perspective, with the notion reported elsewhere regarding the CeO2-doped borate glass that the role of CeO2 has been considered as a network former by forming [CeO4] units at the expense of its role as a network modifier.33,34 Alternatively, cerium ions of a large size and huge mass situated at the interstitial site will occupy much free volume of the network where they are wedged into the space with Na+, K+, Ba2+ seeking optimal close packing, and thus improve structural compactness.
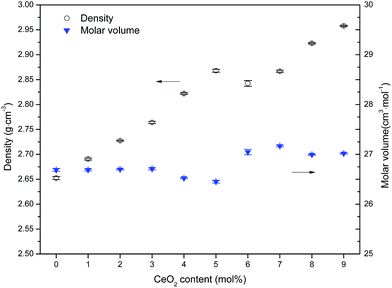
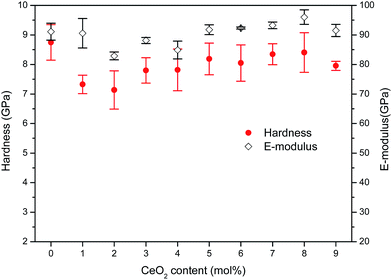
On the whole, doping with CeO2 has three influences on the structure of the studied glass, namely, depolymerization of the borosilicate network, enhanced linkage by charge compensation and improved compactness. The latter two characteristics progressively increase to play the dominant role over the former until settling in the structural state of R > Rd1 corresponding to CeO2 > 3%. For doping with CeO2 levels ranging from 5% to 9%, the relative balance of network connectivity and compactness allows for a mild variation in network rigidness and glass properties. Improved linkage and compactness combined with sharp weight gain cause the glass density to increase upon addition of CeO2. Correspondingly, the molar volume appears to initially increase slightly and then decrease when the CeO2 level exceeds about 3% and tends to stabilize in the range of 5–9%. This structural evolution upon CeO2 addition has also been reflected in mechanical and optical properties that present diverse changing trends under lower and higher CeO2 contents, respectively, because of the inversely fluctuating impact induced by CeO2. There is an intrinsic link between the glass properties and the evolved structural states depending on the content of CeO2 relative to host network formers, and it provides some guidance for developing CeO2-doped boroaluminosilicate glass materials with tailored performances.
5. Conclusion
By factoring multiple oxides including CeO2 into the computation of structural parameter R (the ratio of alkali oxide to B2O3) based on the Yun, Dell and Bray model, the structural states of alkali boroaluminosilicate glass (56SiO2–20B2O3–3Al2O3–8Na2O–8K2O–5BaO) containing 0–9 mol% CeO2 are divided into three categories with 3–4% as the critical content that marks the onset of drastic variations in glass structure and properties. When the added CeO2 levels are below 3%, they are absorbed by reedmergnerite- and danburite-like groups producing one NBO on silica tetrahedrals and disconnecting [BO4] from [SiO4]. Addition of a CeO2 level above 3% allows the additional oxygen to combine with reedmergnerite- and danburite-like units producing two NBOs on [SiO4] and also one or two on [BO3] at the expense of [BO4]. Further adding CeO2 at a level in excess of 5% will make the extra NBOs gradually combine with the disconnected boron triangles which then form boron tetrahedrals in addition to continuous depolymerization of the Si–O network. On the whole, doping CeO2 has three influences on the structural evolution of the studied glass, namely, depolymerization of the skeletal borosilicate network, enhanced linkage by charge compensation and improved compactness because of the close packing, which causes diverse variation trends in the physical properties in the presence of CeO2 levels below and above 3–4%, respectively. The revised structural parameter analysis is explained by the observed structural evolution as well as its correlation with the physical properties, and thus can be a useful reference for the regulation of the constituents of CeO2-doped borosilicate radiation resistant glasses.Acknowledgements
The authors acknowledge the support of Natural Science Foundation of Chongqing (Grant No. cstc2012jjA50034), the Chinese 973 Fundamental Research (2011CB605806) and National Natural Science Foundation of China (50820145202).References
- M. Hubert and A. J. Faber, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2014, 55, 136 CAS.
- J. N. Cachia, X. Deschanels, C. Den Auwer, O. Pinet, J. Phalippou, C. Hennig and A. Scheinost, J. Nucl. Mater., 2006, 352, 182 CrossRef CAS.
- T. Konishi, T. Matsumoto, T. Araki, T. Tsuchiya, S. Todoroki and S. Inoue, Appl. Surf. Sci., 2004, 223, 238 CrossRef CAS.
- S. Peuget, J. M. Delaye and C. Jégou, J. Nucl. Mater., 2014, 444, 76 CrossRef CAS.
- I. W. Donald, B. L. Metcalfe and R. N. J. Taylor, J. Mater. Sci., 1997, 32, 5851 CrossRef CAS.
- N. Baydogan and A. B. Tugrul, Res. Chem. Intermed., 2014, 40, 299 CrossRef CAS.
- R. Kaur, S. Singh and O. P. Pandey, J. Mol. Struct., 2013, 1049, 409 CrossRef CAS.
- R. Kaur, S. Singh and O. P. Pandey, J. Mol. Struct., 2013, 1049, 386 CrossRef.
- M. Arora, S. Baccaro, G. Sharma, D. Singh, K. S. Thind and D. P. Singh, Nucl. Instrum. Methods Phys. Res., Sect. B, 2009, 267, 817 CrossRef CAS.
- G. A. Haynes, NASA technical note, National aeronautics and space administration, Washington, DC, 1970, p. 1 Search PubMed.
- A. Paul, M. Mulholland and M. S. Zaman, J. Mater. Sci., 1976, 11, 2082 CrossRef CAS.
- Y. Sheng, L. Yang, H. Luan, Z. Liu, Y. Yu, J. Y. Li and N. Dai, J. Nucl. Mater., 2012, 427, 58 CrossRef CAS.
- B. Speit, E. Radlein, G. H. Frischat, A. J. Marker and J. S. Hayden, Nucl. Instrum. Methods Phys. Res., Sect. B, 1992, 65, 384 CrossRef.
- Z. Han, Z. Tang, Q. Chen, X. Gong and H. Zhao, J. Chin. Rare Earth Soc., 2010, 28, 275 Search PubMed.
- T. D. Henson and G. K. Torrington, Proc. SPIE, 2001, 4452, 54 CrossRef CAS.
- V. K. Deshpande and R. N. Taikar, Mater. Sci. Eng., B, 2010, 172, 6 CrossRef.
- Y. H. Yun and P. J. Bray, J. Non-Cryst. Solids, 1978, 27, 363 CrossRef.
- Y. H. Yun, S. A. Feller and P. J. Bray, J. Non-Cryst. Solids, 1979, 33, 273 CrossRef CAS.
- W. J. Dell, P. J. Bray and S. Z. Xiao, J. Non-Cryst. Solids, 1983, 58, 1 CrossRef.
- S. Wang and J. F. Stebbins, J. Non-Cryst. Solids, 1998, 231, 286 CrossRef CAS.
- D. Manara, A. Grandjean and D. R. Neuville, J. Non-Cryst. Solids, 2009, 355, 2528 CrossRef CAS.
- A. Rupesh Kumar, T. G. V. M. Rao, K. Neeraja, M. Rami Reddy and N. Veeraiah, Vib. Spectrosc., 2013, 69, 49 CrossRef CAS.
- P. Trocellier, A. Haddi, S. Poissonnet, P. Bonnaillie and Y. Serruys, Nucl. Instrum. Methods Phys. Res., Sect. B, 2006, 249, 145 CrossRef CAS.
- G. Yang, G. Mobus and R. J. Hand, Micron, 2006, 37, 433 CrossRef CAS PubMed.
- C. Gautam, A. K. Yadav and A. K. Singh, ISRN Ceram., 2012, 2012, 1 CrossRef.
- A. K. Yadav and P. Singh, RSC Adv., 2015, 5, 67583 RSC.
- S. L. Lin and C. S. Hwang, J. Non-Cryst. Solids, 1996, 202, 61 CrossRef CAS.
- M. R. Adams, R. Engelmann, P. D. Grannis, J. Horstkotte, L. Godfrey, S. L. Linn, M. D. Marx, J. Timms, P. M. Tuts and J. Willins, Nucl. Instrum. Methods Phys. Res., Sect. A, 1985, 238, 333 CrossRef.
- P. Kaur, G. P. Singh, S. Kaur and D. P. Singh, J. Mol. Struct., 2012, 1020, 83 CrossRef CAS.
- G. P. Singh, P. Kaur, S. Kaur and D. P. Singh, Phys. B, 2012, 407, 4168 CrossRef.
- Y. M. Lai, X. F. Liang, S. Y. Yang, J. X. Wang, L. H. Cao and B. Dai, J. Mol. Struct., 2011, 992, 84 CrossRef CAS.
- J. Du and L. Kokou, J. Am. Ceram. Soc., 2011, 94, 2393 CrossRef CAS.
- G. El-Damrawi and K. El-Egili, Phys. B, 2001, 299, 180 CrossRef CAS.
- E. Mansour, J. Non-Cryst. Solids, 2011, 357, 1364 CrossRef CAS.
- Y. Y. Ivanova, E. J. Spassova, E. P. Kashchieva, M. A. Bursukova and Y. B. Dimitriev, J. Non-Cryst. Solids, 1995, 192–193, 674 CrossRef.
- Q. Zhao, M. Guerette and L. Huang, J. Non-Cryst. Solids, 2012, 358, 652 CrossRef CAS.
- X. Li, L. Jiang, X. Zhang and Y. Yan, J. Non-Cryst. Solids, 2014, 385, 1 CrossRef CAS.
- W. C. Oliver and G. M. Pharr, J. Mater. Res., 2004, 19, 3 CrossRef CAS.
- G. M. Pharr, Mater. Sci. Eng., A, 1998, 253, 151 CrossRef.
- A. M. Efimov, V. G. Pogareva and A. V. Shashkin, J. Non-Cryst. Solids, 2003, 332, 93 CrossRef CAS.
- R. A. Brooker, S. C. Kohn, J. R. Holloway and P. F. McMillan, Chem. Geol., 2001, 174, 241 CrossRef CAS.
- A. K. Sandhu, S. Singh and O. P. Pandey, Mater. Chem. Phys., 2009, 115, 783 CrossRef CAS.
- E. Mansour, J. Non-Cryst. Solids, 2012, 358, 454 CrossRef CAS.
- C. R. Gautam, D. Kumar, O. M. Parkash and P. Singh, Bull. Mater. Sci., 2013, 36, 461 CrossRef CAS.
- C. R. Gautam, D. Kumar and O. M. Parkash, Bull. Mater. Sci., 2010, 33, 145 CrossRef CAS.
- M. Massot, C. Julien and M. Balkanski, Infrared Phys., 1989, 29, 775 CrossRef CAS.
- M. S. Gaafar and S. Y. Marzouk, Phys. B, 2007, 388, 294 CrossRef CAS.
- G. H. Kim and I. Sohn, J. Non-Cryst. Solids, 2012, 358, 1530 CrossRef CAS.
- Y. Cheng, H. Xiao, S. Chen and B. Tang, Phys. B, 2009, 404, 1230 CrossRef CAS.
- E. I. Kamitsos, M. A. Karakassides and G. D. Chryssikos, J. Phys. Chem., 1987, 91, 5807 CrossRef CAS.
- S. Song, Z. Wen, Y. Liu, Q. Zhang, X. Wu, J. Zhang and J. Han, Ceram. Int., 2009, 35, 3037 CrossRef.
- J. Bonfils, S. Peuget, G. Panczer, D. Ligny, S. Henry, P. Y. Noël, A. Chenet and B. Champagnon, J. Non-Cryst. Solids, 2010, 356, 388 CrossRef.
- D. Manara, A. Grandjean and D. R. Neuville, Am. Mineral., 2009, 94, 777 CrossRef CAS.
- V. E. Eremyashev, A. A. Osipov and L. M. Osipova, Glass Ceram., 2011, 68, 205 CrossRef CAS.
- O. N. Koroleva, L. A. Shabunina and V. N. Bykov, Glass Ceram., 2011, 67, 340 CrossRef CAS.
- A. G. Kalampounias, Bull. Mater. Sci., 2011, 34, 299 CrossRef CAS.
- T. Yano, S. Shibata and T. Maehara, J. Am. Ceram. Soc., 2006, 89, 89 CrossRef CAS.
- Y. Ou, S. Baccaro, Y. Zhang, Y. Yang and G. Chen, J. Am. Ceram. Soc., 2010, 93, 338–341 CrossRef CAS.
- A. F. Maged and F. Abdel Rehim, Appl. Radiat. Isot., 1991, 42, 763 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |