Enhanced transport of novel crystalline calcium-phosphonate scale inhibitor nanomaterials and their long term flow back performance in laboratory squeeze simulation tests†
Abstract
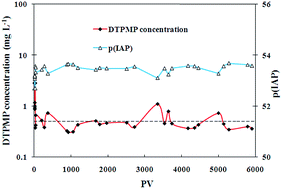
In this study, novel crystalline phase calcium-phosphonate scale inhibitor nanomaterials were prepared from amorphous silica templated calcium-phosphonate precipitates. The transport of the nanomaterial suspension (nanofluid) was investigated in calcium carbonate and sandstone formation media using laboratory column breakthrough experiments. The nanomaterials were transportable through these formation media and the transport data can be interpreted using an advection–dispersion equation and a classical colloidal filtration theory. By preflushing the formation media prior to nanofluid injection, the nanofluid experienced enhanced migration in the column breakthrough tests. This observation can be explained by the calculation results of interaction energy of the nanomaterials with the formation medium particles by using Derjaguin–Landau–Verwey–Overbeek (DLVO) theory. The long term flow back performance of the crystalline nanomaterials was evaluated in laboratory squeeze simulation tests where the crystalline materials were first attached to the formation medium surfaces and then gradually returned phosphonates into the brine solution during flow back. Due to the low solubility of the crystalline nanomaterials, a long return profile with relatively stable phosphonate return concentrations can be observed in both calcium carbonate and sandstone media, suggesting of the potential advantage of applying these crystalline inhibitor nanomaterials in oilfield operations.

 Please wait while we load your content...
Please wait while we load your content...