Washing pretreatment with light bio-oil and its effect on pyrolysis products of bio-oil and biochar
Abstract
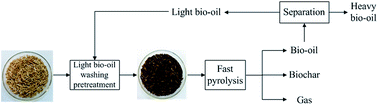
Rice husk is an abundant agricultural waste and the method commonly used for the disposal of rice husk could cause serious environmental and human health problems. Herein, an attempt is made to convert rice husk into value added bio-based products, such as bio-oil and biochar, using washing pretreatment with light bio-oil followed by fast pyrolysis. It can be found that the washing pretreatment with light bio-oil effectively removes a large amount of alkali and alkaline earth metals (AAEMs), and has higher removal efficiency than aqueous HCl at the same pH of 2.8 because the phenolic compounds in light bio-oil promote the removal of some AAEMs. Furthermore, the pyrolysis of washed rice husk with light bio-oil results in an increase in bio-oil yield and decrease in water and biochar yields. GC/MS analysis indicated that light bio-oil washing pretreatment prior to a fast pyrolysis process has an important effect on the quality of bio-oil, which results in a significant increase in the relative content of levoglucosan in bio-oil along with a reduction of low molecular weight compounds. Biochar, which is produced in higher yield, has a high silica content and high surface area, and has the potential to produce amorphous silica as well as adsorbent or catalyst support. This study reveals a promising process to convert biomass waste into quality bio-based products.

 Please wait while we load your content...
Please wait while we load your content...