Robust carboxylated polymer pores from a cyclic peptide template†
Mahesh
Potnuru
and
Nandita
Madhavan
*
Department of Chemistry, Indian Institute of Technology, Madras, Chennai, Tamil Nadu 600 036, India. E-mail: nanditam@iitm.ac.in; Fax: (+91)-44-22574202
First published on 21st October 2015
Abstract
Functionalized pores that are stable to chemical degradation are attractive for use in a variety of applications ranging from sensing to catalysis. Herein, robust polymer nanotube bundles decorated with carboxylate groups inside the pores have been developed in two steps from a cyclic peptide template containing polymerizable norbornene units. The cyclic peptide forms nanotube bundles of ca. 35 nm in diameter. Polymerization of the exposed norbornene units gives peptide–polymer hybrid nanotube bundles of ca. 100 nm diameter that have improved thermal and chemical stability compared to cyclic peptide nanotubes. Hydrolysis of the ester linkage connecting norbornene to the peptide scaffold and removal of the peptide provide robust polymer pores of ca. 100 nm that are not only chemically stable, but also have carboxylate groups in the pores. Such nanotube bundles with carboxylate groups can be potentially used for encapsulation of cations or biomimetic ion transport.
Organic nanotubes are emerging as useful soft materials because their properties can be readily tuned by varying their pore and surface functional groups. Functionalized nanotubes have been used for a variety of applications ranging from molecular encapsulation to tissue engineering.1,2 Cyclic peptides comprising an even number of alternating L and D amino acids are known to form nanotubes.3,4 These cyclic peptides have a flat ring-like conformation with the backbone amide bonds perpendicular to the plane of the ring and the amino acid side chains projecting outwards. Such a unique conformation allows the formation of nanotubes that are stabilized by backbone hydrogen bonding interactions between the stacked peptides. These peptide nanotubes with amino acid side chains decorating their external surface have been used as ion channel mimics,5–7 antibacterial agents8,9 and sensors.10,11 Polymer chains have been appended onto the external surface of the peptide nanotubes to improve their solubility.12–18 However, the supramolecular cyclic peptide assembly loses its structural integrity in the presence of strong hydrogen bonding donors or acceptors. Enhancing the stability of these nanotubes is desirable for practical applications. The stability of nanotubes has been improved by crosslinking the polymer chains on the peptide–polymer conjugates.19 For applications such as molecular encapsulation/recognition and water purification it is advantageous to develop robust functionalized pores or membrane like aggregates. Since the amino acids radiate outwards from cyclic peptide nanotubes it has been challenging to functionalize their pore interior. Functionalized tubes have been developed from cyclic peptides appended with block copolymers obtained from t-butyl acrylate and isoprene.20 Seven synthetic steps (excluding the cyclic peptide synthesis) were required to obtain these functionalized pores.
Herein, we propose a synthetic route to access robust functionalized polymer nanotube bundles in two steps from cyclic peptide 1 appended with norbornene units (Fig. 1). Cyclic peptide nanotubes derived from alternating L and D amino acids (without polymer chains attached) are reported to form bundles of nanotubes.21,22 Polymerization of the exposed norbornene groups in the presence of a dinorbornene crosslinker 2 gives nanotube bundles where the covalent bonds from the polymer backbone reinforce the stability of the cyclic peptide assembly. Hydrolysis of the ester linkages connecting the cyclic peptide to the polymer provides large robust pores or bundles of pores lined with carboxylate groups.
Cyclic peptide 1 appended with norbornene units at the L amino acids was chosen as the pore template as norbornene is reported to undergo ring opening metathesis polymerization (ROMP) readily.23 A flexible linker was incorporated in the dinorbornene crosslinker 2 to ensure that it can react with the norbornene monomers positioned longitudinally or laterally on the surface of the self-assembled cyclic peptide nanotube bundles.
Cyclic peptide 1 was synthesized from the appropriate L serine and D alanine derivatives (Scheme 1). Protected serine derivative 3 was treated with norbornene-exo-acid, EDC and N,N-dimethylamino pyridine (DMAP) to afford the ester in 88% yield. TFA deprotection gave serine 4, which was coupled with Boc-D-alanine in the presence of O-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU) and N,N-diisopropylethyl amine (DIEA) to give dipeptide 5 in 92% yield. The dipeptide 5 was converted to the acid 6 in 87% yield using tetrakis(triphenylphosphine)palladium, triphenylphosphine and pyrrolidine. The dipeptide 7 with the free amine was obtained by treating dipeptide 5 with TFA. Dipeptides 6 and 7 were coupled in the presence of HCTU and DIEA to give tetrapeptide 8 in 74% yield. The above mentioned deprotection and coupling steps were carried out with tetrapeptide 8 to afford octapeptide 11 in 52% yield. Octapeptide 11 was deprotected and subsequently cyclized using HCTU and DIEA. The crude cyclic peptide 1 obtained from the reaction mixture as a precipitate with water was washed with water and methanol to afford pure peptide 1 in 68% yield.
Peptide 1 was allowed to self-assemble in solution for 5 days. The formation of nanotube bundles with an average diameter of 36 nm (standard deviation 18, statistical analysis of 130 tubes) was confirmed from the TEM images (Fig. S1† & 2a). The large standard deviation is because the size distribution of nanotubes was found to be non-Gaussian. Among the 130 tubes analysed, 101 had an average diameter of 22 nm (std dev. 6), while the remaining 29 had an average diameter of 55 nm (std dev. 9). The nanotube bundles were polymerized with varying concentration of cross-linker 2 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 DMF–THF using Grubbs’ 2nd generation initiator to determine the optimal reaction conditions for obtaining the polymer peptide conjugates (PPC) 12 (Scheme 1, Table S1†). The reactions were carried out where the initiator was slowly added to the cyclic peptide solution. Subsequently the crosslinker 2 was slowly added to the reaction mixture. The slow-addition as well as a large monomer
4 DMF–THF using Grubbs’ 2nd generation initiator to determine the optimal reaction conditions for obtaining the polymer peptide conjugates (PPC) 12 (Scheme 1, Table S1†). The reactions were carried out where the initiator was slowly added to the cyclic peptide solution. Subsequently the crosslinker 2 was slowly added to the reaction mixture. The slow-addition as well as a large monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator ratio were used to minimize non-templated polymerization. Furthermore, in all reactions a 1
initiator ratio were used to minimize non-templated polymerization. Furthermore, in all reactions a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cross-linker to monomer ratio was used to ensure that each cross-linker unit can react with two neighbouring units of norbornene on a nanotube bundle. The crude PPC 12 was isolated as a precipitate in methanol and sequentially washed with water and methanol to remove lower molecular weight polymers and the starting materials.
1 cross-linker to monomer ratio was used to ensure that each cross-linker unit can react with two neighbouring units of norbornene on a nanotube bundle. The crude PPC 12 was isolated as a precipitate in methanol and sequentially washed with water and methanol to remove lower molecular weight polymers and the starting materials.
We observed that at high solvent concentrations distinct nanotubes were not seen and extended network like structures were observed (Table S1, Fig. S2†). At a higher dilution without and with a cross-linker (entries 7 & 8, Table S2) PPCs 12g and 12h, respectively, were obtained which did not show extended network like structures.24 The SEM and TEM images of 12g only showed a couple of nanotubes (Fig. S2†). The images of PPC 12h showed nanotube bundles (Fig. 2b) with an average diameter of 98 nm (standard deviation 21, statistical analysis of 105 tubes). The 2–3 fold increase in the diameter could be attributed to the polymer coating on these nanotube bundles. Such an increase in the diameter of the cyclic peptide nanotubes has been observed for non-crosslinked peptide polymer conjugates.16 Alternatively, it can indicate the formation of cross-links between multiple nanotube bundles. PPCs 12g and 12h were also characterized by IR spectroscopy (Fig. S5†).25
Dynamic light scattering (DLS) was used to check the stability of PPC 12h after treatment with trifluoroacetic acid (TFA).26 Relative size distribution of peptide 1 and cross-linked peptide nanotubes 12h was studied by DLS. In 5% DMF/THF, cyclic peptide 1 formed nanotubes with a mean radius of ca. 118 nm and a count rate of 140 kcps. When the cyclic peptide solution was concentrated and re-dispersed in DMF/TFA (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the mean radius reduced drastically to 11–14 nm and the count rate dropped to 50 kcps (Fig. 3a). In the case of PPC 12h the mean radius of ∼450 nm did not change upon adding TFA (Fig. 3b). The reduction in the count rate from ∼260 to 180 kcps was also smaller. Similar behavior was observed with PPC 12g (Fig. S3†). The thermal stability of PPC 12h was assessed using TGA. Distinct higher Td peaks are observed in 12h compared to peptide 1 (Fig. 3c and d). Furthermore, comparison of the TGA of PPCs 12g (Fig. S4†) and 12h indicates that PPC 12h obtained in the presence of external crosslinker 2 is more thermally stable than 12g.
1), the mean radius reduced drastically to 11–14 nm and the count rate dropped to 50 kcps (Fig. 3a). In the case of PPC 12h the mean radius of ∼450 nm did not change upon adding TFA (Fig. 3b). The reduction in the count rate from ∼260 to 180 kcps was also smaller. Similar behavior was observed with PPC 12g (Fig. S3†). The thermal stability of PPC 12h was assessed using TGA. Distinct higher Td peaks are observed in 12h compared to peptide 1 (Fig. 3c and d). Furthermore, comparison of the TGA of PPCs 12g (Fig. S4†) and 12h indicates that PPC 12h obtained in the presence of external crosslinker 2 is more thermally stable than 12g.
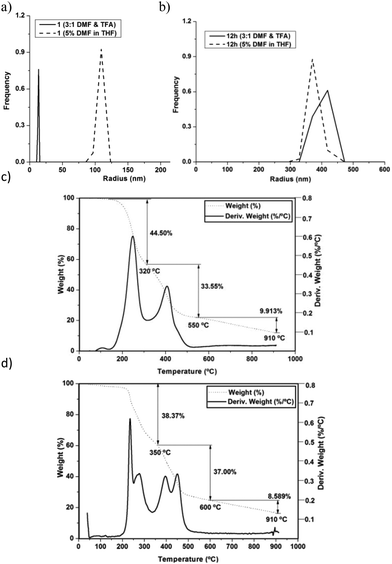 | ||
| Fig. 3 Stability cyclic peptide 1 and PPC 12h nanotubes towards (a) and (b) TFA treatment; (c) and (d) thermal decomposition. | ||
Functionalized polymeric pores 13 were obtained by treating a solution of PPC 12h with an excess of LiOH·H2O over a period of 2 days (eqn (1)). FP 13 was separated from the reaction mixture as a precipitate with methanol. FP 13 was washed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TFA/water (3 × 5 mL) and 1
1 TFA/water (3 × 5 mL) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TFA/methanol (3 × 5 mL) to remove the peptide template and excess reagents.
1 TFA/methanol (3 × 5 mL) to remove the peptide template and excess reagents.
 | (1) |
SEM images of FP 13 (Fig. S6†) obtained after washing with methanol showed that the polymer nanotube bundles retained their structural integrity after hydrolysis. Aggregates of non-specific shape were also observed in addition to the functionalized pores. These could be attributed to the incomplete removal of the peptide template. FP 13 was re-dispersed in 5 mL of DMF/TFA (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to destroy the peptide aggregates and subsequently characterized by TEM. The TEM images (Fig. 4a) showed that the structural integrity of the nanotubes was intact despite the multiple treatments with TFA. The average diameter of the nanotube bundles was found to be 96 nm (standard deviation 17, statistical analysis of 90 tubes) similar to PPC 12. We believe that the hydrolysis of the PPC leads to the formation of hollow nanotube bundles. The interface between the individual nanotubes is difficult to observe in the bundles because of the lower polymer density at these less accessible regions. DLS studies also confirmed that FP 13 tubes maintained their structural integrity upon addition of TFA (Fig. 4b). The mean radius of 550 nm as well as the count rate of 110 kcps did not decrease upon the addition of TFA. FP 13 was also characterized by IR spectroscopy. Comparison of the IR spectra of FP 13 and PPC 12h indicated a shift in the C
1 v/v) to destroy the peptide aggregates and subsequently characterized by TEM. The TEM images (Fig. 4a) showed that the structural integrity of the nanotubes was intact despite the multiple treatments with TFA. The average diameter of the nanotube bundles was found to be 96 nm (standard deviation 17, statistical analysis of 90 tubes) similar to PPC 12. We believe that the hydrolysis of the PPC leads to the formation of hollow nanotube bundles. The interface between the individual nanotubes is difficult to observe in the bundles because of the lower polymer density at these less accessible regions. DLS studies also confirmed that FP 13 tubes maintained their structural integrity upon addition of TFA (Fig. 4b). The mean radius of 550 nm as well as the count rate of 110 kcps did not decrease upon the addition of TFA. FP 13 was also characterized by IR spectroscopy. Comparison of the IR spectra of FP 13 and PPC 12h indicated a shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peaks due to the loss of the ester group (Fig. S5†). We compared the IR spectra of FP 13 with a crosslinked polymer 14 prepared from norbornene-exo-acid and crosslinker 2. Similar C
O stretching peaks due to the loss of the ester group (Fig. S5†). We compared the IR spectra of FP 13 with a crosslinked polymer 14 prepared from norbornene-exo-acid and crosslinker 2. Similar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peaks were observed for these polymers (Fig. S5†).
O stretching peaks were observed for these polymers (Fig. S5†).
 | ||
Fig. 4 (a) TEM image of FP 13 (0.1 mg per 5 mL in DMF/TFA (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 V/V)) (b) DLS of FP 13 indicating its stability. 1 V/V)) (b) DLS of FP 13 indicating its stability. | ||
To check whether the pores are hollow and carboxylate ions are present inside the pores a cationic dye lucigenin was encapsulated in FP 13. To accomplish this, a solution of FP 13 was allowed to stir for 14 hours in the presence of lucigenin. The dye incorporated FP 13 was recovered as a precipitate and washed with methanol (10 times) to remove the free dye. Fluorescence images of the resultant nanotubes indicated incorporation of lucigenin (Fig. 5a and S8†). Bundling can be observed in the bright field image (Fig. S8†). As a control experiment, encapsulation of cationic lucigenin was attempted with PPC 12h. The images (Fig. 5b and S7†) indicated that lucigenin was not bound to the uncharged PPC 12h. Hence, we believe that the electrostatic interaction between the carboxylate groups in FP 13 and the cationic dye provides the driving force for dye encapsulation. In order to rule out the presence of carboxylate groups on the pore exterior, the zeta potential of FP 13 was determined using electrophoretic light scattering. The pH of the solution was maintained at 7.2 to ensure that the carboxylic acid groups were ionized. The zeta potential was found to be −2.1 mV indicating that there was no significant negative charge at the external surface of the nanotubes (Fig. 5c). Based on the dye encapsulation studies with PPC 12h and the zeta potential studies, we conclude that the pore interior of FP 13 is negatively charged.
 | ||
| Fig. 5 (a) Fluorescent images of dye incorporation studies with (a) FP 13; (b) PPC 12h; (c) zeta potential measured for FP 13. | ||
Conclusions
In conclusion, carboxylated pores were obtained from a cyclic peptide appended with norbornene units. The cyclic peptide formed nanotube bundles that could be polymerized under high dilution in the presence of a crosslinker to give nanotubes of larger diameter. These peptide–polymer nanotubes were characterized by microscopy, IR spectroscopy, TGA and DLS. TGA and DLS studies showed that the PPCs were more stable than the cyclic peptide nanotube bundles. The peptide–polymer conjugates were hydrolyzed to remove the templates and provide pores functionalized with carboxylic acid groups in the interior. The functionalized pores were characterized by IR, SEM and TEM. DLS and TEM studies indicated that the functionalized pores are stable to TFA treatment. A positively charged fluorescent dye was encapsulated inside these functionalized pores. The minimal zeta potential of the pores as well as the inability of the peptide polymer conjugates to encapsulate the cationic dye indicates that the pore interior is negatively charged. Current efforts are focused on modifying the functional groups inside the pore and expanding the utility of these pores.Acknowledgements
This research was supported by DST (SR/S1/OC-41/2009), New Delhi, India. M. P. acknowledges CSIR, India for his research fellowship. We thank Mr Narayanan for help with the TGA experiments. We thank Dr E. Prasad and Dr Basavaraj M. G. for the use of their DLS instruments. We thank Dr Madhulika Dixit and Dr Basavaraj M. G. for the use of their fluorescence microscopes. We thank the Sophisticated Analytical Instruments Facility (SAIF) and Dept. of Metallurgical and Materials Engineering, IIT Madras for SEM and TEM images.Notes and references
- N. Kameta, H. Minamikawa and M. Masuda, Soft Matter, 2011, 7, 4539 RSC.
- T. Shimizu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2601 CrossRef CAS.
- D. T. Bong, T. D. Clark, J. R. Granja and M. R. Ghadiri, Angew. Chem., Int. Ed., 2001, 40, 988 CrossRef CAS.
- R. J. Brea, C. Reiriz and J. R. Granja, Chem. Soc. Rev., 2010, 39, 1448 RSC.
- J. Montenegro, M. R. Ghadiri and J. R. Granja, Acc. Chem. Res., 2013, 46, 2955 CrossRef CAS PubMed.
- R. Garcia-Fandino, M. Amorin, L. Castedo and J. R. Granja, Chem. Sci., 2012, 3, 3280 RSC.
- M. R. Ghadiri, J. R. Granja and L. K. Buehler, Nature, 1994, 369, 301 CrossRef CAS PubMed.
- S. Fernandez-Lopez, H. S. Kim, E. C. Chol, M. Delgado, J. R. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D. A. Weinberger, K. M. Wilcoxen and M. R. Ghadiri, Nature, 2001, 414, 329 CrossRef CAS.
- J. T. Fletcher, J. A. Finlay, M. E. Callow, J. A. Callow and M. R. Ghadiri, Chem. – Eur. J., 2007, 13, 4008 CrossRef CAS PubMed.
- J. Sanchez-Quesada, M. R. Ghadiri, H. Bayley and O. Braha, J. Am. Chem. Soc., 2000, 122, 11757 CrossRef CAS.
- K. Motesharei and M. R. Ghadiri, J. Am. Chem. Soc., 1997, 119, 11306 CrossRef CAS.
- R. Chapman, K. A. Jolliffe and S. Perrier, Polym. Chem., 2011, 2, 1956 RSC.
- H. G. Börner, Macromol. Chem. Phys., 2007, 208, 124 CrossRef.
- S. Dehn, R. Chapman, K. A. Jolliffe and S. Perrier, Polym. Rev., 2011, 51, 214 CrossRef CAS.
- J. Couet and M. Biesalski, Small, 2008, 4, 1008 CrossRef CAS PubMed.
- J. Couet, J. Samuel, A. Kopyshev, S. Santer and M. Biesalski, Angew. Chem., Int. Ed., 2005, 44, 3297 CrossRef CAS PubMed.
- R. Chapman, K. A. Jolliffe and S. Perrier, Aust. J. Chem., 2010, 63, 1169 CrossRef CAS.
- S. Loschonsky, J. Couet and M. Biesalski, Macromol. Rapid Commun., 2008, 29, 309 CrossRef CAS.
- R. Gokhale, J. Couet and M. Biesalski, Phys. Status Solidi A, 2010, 207, 878 CrossRef CAS.
- R. Chapman, K. A. Jolliffe and S. Perrier, Adv. Mater., 2013, 25, 1170 CrossRef CAS PubMed.
- J. D. Hartgerink, J. R. Granja, R. A. Milligan and M. R. Ghadiri, J. Am. Chem. Soc., 1996, 118, 43 CrossRef CAS.
- J. M. Buriak and M. R. Ghadiri, Mater. Sci. Eng., C, 1997, 4, 207 CrossRef.
- T. L. Choi and R. H. Grubbs, Angew. Chem., Int. Ed., 2003, 115, 1785 CrossRef.
- Concentrations below 0.2 mM were not explored for polymerization.
- The PPCs 12g and 12h were found to be marginally soluble in DMF. Therefore, they could not be characterized by NMR.
- Dilute solutions of peptide 1 and PPCs 12 were used as the nanotubes were not soluble in lower amounts of solvent.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, spectral data of compounds, polymerization conditions and additional material characterization. See DOI: 10.1039/c5py01313e |
| This journal is © The Royal Society of Chemistry 2016 |



