A pH-responsive sequential-disassembly nanohybrid for mitochondrial targeting†
Lijia
Li
,
Wei
Sun
,
Lian
Li
,
Yuanyuan
Liu
,
Lei
Wu
,
Fengling
Wang
,
Zhou
Zhou
,
Zhirong
Zhang
and
Yuan
Huang
*
Key Laboratory of Drug Targeting and Drug Delivery System, Ministry of Education, West China School of Pharmacy, Sichuan University, No. 17, Block 3, Southern Renmin Road, Chengdu 610041, PR China. E-mail: huangyuan0@163.com
First published on 4th November 2016
Abstract
Cationic materials have been reported as promising tools for targeting to mitochondria which are the “power houses” and “metabolic garbage keepers” of cells. However, their positive nature also restricts their in vivo application due to the quick clearance. Herein, we fabricated a nanohybrid consisting of the pH-responsive N-(2-hydroxypropyl)methacrylamide (HPMA) co-polymer (R-P) shells and positive mesoporous silica nanoparticle cores via electrostatic interaction. The anticancer drug, docetaxel (DTX), was encapsulated in the positive MSN cores (MSN–DTX). Once concealed by the anionic R-P shield, the assembled nanohybrid R-P@MSN–DTX will achieve prolonged blood circulation thereby leading to an enhanced EPR effect. At mildly acidic tumor environmental pH, first-stage charge reversion took place due to the hydrolysis of the amide bond on HPMA co-polymers. The de-attachment of the HPMA co-polymer occurred because of the positive charge repulsion and partial exposure of the positively charged MSN core promoted the cell internalization. The second-stage pH-responsiveness in the endo/lysosomes with a more acidic environment accelerates the disassembly of the nanohybrid and the leakage of the core facilitated the endo/lysosome escape and mitochondrial targeting with the help of intracellular compartmental acidity. Gathering up the characteristics of neutralized charge and stepwise pH-responsiveness, the R-P@MSN–DTX acquired a good tumor inhibition rate of 72.6% on nude mice. Our report provided a reference for systemic mitochondrial targeting achieved by the union of “assembly–disassembly”.
1. Introduction
Mitochondria, the membrane-bound organelles, act as “power houses” where the aerobic respiration happens,1,2 yet, they are so much more—they are “renaissance organelles” that work in anfractuous networks to fulfil crucial cellular tasks such as cell division.3 Therefore, mitochondrial dysfunction is a hallmark for a series of diseases including obesity, diabetes, Alzheimer's disease and cancer.4–7 Because of the close relationship between cell death and mitochondria of cancer cells,8 delivering anticancer drugs into mitochondria is an urgent task.Mitochondrial targeting is based on the high negative potential of the inner mitochondrial membrane.9 In the early studies, drugs, such as α-tocopheryl succinate or the anthracycline antitumor agent doxorubicin (DOX), were directly conjugated to the classic mitochondriotropic ligand triphenylphosphonium (TPP) to construct a prodrug.10,11 Hence, the sufficient delocalization of the positive charge of the TPP realized the mitochondrial specific drug delivery in response to the mitochondrial membrane potential.12,13 However, an insufficient circulation half-life period owing to the positive charge and low drug loading capacity limits their use in mitochondrial drug delivery.
A nano-sized drug delivery system (DDS), which could incorporate drugs inside the vector, may be a sensible choice for mitochondrial targeting. Decorated with cationic mitochondrial targeting anchors including the lipophilic cationic ligand TPP or a zwitterionic oligopeptide,14 many DDSs such as HPMA co-polymers,15,16 liposomes17,18 and polymeric nanoparticles9,14,19 have been fabricated to deliver drugs into the mitochondria. However, the drug loading capacity was still less than satisfactory. Therefore, it is essential to fabricate a mitochondrial targeting DDS possessing a higher drug loading, longer circulation half-life period and exposing positive charge on demand.
The superiority of large loading capacity, good biocompatibility, and versatile surface functionalization of mesoporous silica nanoparticles make them promising carriers to meet the demand.20–22 Also, the positive charge could be fulfilled by chemical modification thus to conquer both barriers of the cell membrane and mitochondrial membrane,3i.e. kill two birds with one stone. Meanwhile, once the drugs are loaded into the pores, the MSNs will act as a “shelter” compared with the prodrug, protecting the drug from degradation before release at mitochondria.
However, the positive charge makes the MSNs hindered by the reticuloendothelial system (RES) once injected into blood.23 Thus, it is not surprising that efficient endocytosis is conflicting with long circulation and sufficient tumor accumulation for cationic mesoporous silica nanoparticles. Could the cationic mesoporous silica nanoparticles transform the conflict into cooperation?
The N-(2-hydroxypropyl)methacrylamide (HPMA) co-polymer, possessing non-immunogenicity, non-toxicity, biocompatibility and multifunctionality,24–26 has been proven to be an attractive candidate for polyethylene glycol (PEG).27 Herein, to meet the conflicting requirements for efficient mitochondrial targeting, we proposed a charge-reversible HPMA-coated mesoporous silica NP based nanohybrid for mitochondrial targeting. As illustrated in Scheme 1, the core–shell nanohybrid is formed from two parts: (a) the cationic MSN cores; (b) anionic 2,3-dimethylmaleic anhydride (DMA) modified HPMA co-polymer shells. After being attracted by the opposite charges between the core and the shell, plus the physical entanglement among polymers,28 a negatively-charged nanohybrid was constructed. Compared with the bare positive MSN, the charge-shielding nanohybrid will avoid being quickly cleared out, achieve long blood circulation and finally reach the tumor environment via the enhanced permeation and retention (EPR) effect. Notably, the HPMA chain modified by DMA was not trustworthy28 which would be sequentially cleaved along with the multistep pH degradation from the tumor microenvironment (pH 6.5) to the endo/lysosomes (pH 5.0). So, the gradual re-exposure of the positively charged MSN core will simultaneously facilitate the endocytosis and endo/lysosome escape for mitochondriotropic drug delivery. Meanwhile, docetaxel with a better therapeutic efficiency than paclitaxel, which was proven to depolarize mitochondria, reduces the mitochondrial membrane potential and increases the permeability of the mitochondria,18,29 was employed as the model drug.
2. Materials and methods
2.1 Materials
Tetraethyl orthosilicate (TEOS, 98%) and cetyltrimethylammonium bromide (CTAB) were acquired from Aladdin Industrial Inc. (Shanghai, China). 3-Aminopropyltriethoxysilane (APTES) was obtained from Meryer Chemical Technology Co. Ltd (Shanghai, China). Docetaxel (DTX) was bought from Dalian Meilun Biotech Co. Ltd (Shandong, China). Cyanine 5 NHS ester (Cy5-NHS) was purchased from Lumiprobe (FL, USA). N-3-Aminopropylmethacrylamide hydrochloride (APMA) was purchased from PolyScience (IL, USA). DMA was obtained from Acros Organics (NJ, USA). 4,6-Diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate (FITC), and 3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were all obtained from Sigma-Aldrich (St Louis, MO). The bicinchoninic acid (BCA) kit was purchased from KeyGEN Inc. (Nanjing, China). MitoTracker Red was obtained from Invitrogen (Eugene, OR, USA). The ROS detection kit and JC-1 molecular probe were bought from the Beyotime Institute of Biotechnology (Haimen, China). All other chemicals and reagents were of analytical grade.2.2 Synthesis and characterization of various monomers and polymers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA-GG-OH = 80
MA-GG-OH = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, respectively). Co-polymerization was carried out in sealed ampoules under nitrogen at 50 °C for 24 h. The co-polymer was isolated from the polymerization mixture by precipitating into diethyl ether, and then purified by dialysis against distilled water and freeze-dried.
20, respectively). Co-polymerization was carried out in sealed ampoules under nitrogen at 50 °C for 24 h. The co-polymer was isolated from the polymerization mixture by precipitating into diethyl ether, and then purified by dialysis against distilled water and freeze-dried.
The DMA-modified pH-responsive HPMA co-polymer (R-P) HPMA–DMA was synthesized in two steps as reported.28 Briefly, the polymer precursor HPMA–APMA with abundant positively charged amine groups was prepared by radical solution polymerization in absolute methanol (AIBN, 2 wt%; monomer concentration 12.5 wt%; molar ratio HPMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APMA = 80
APMA = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, respectively). Co-polymerization was carried out in sealed ampoules with the protection of nitrogen at 50 °C for 24 h. The co-polymer was isolated from the polymerization mixture by precipitating into diethyl ether, purified by dialysis against distilled water and then freeze-dried. The HPMA–APMA co-polymer was dissolved in sodium bicarbonate buffer (0.1 M, pH 8.5) and DMA (3 molar equivalents to APMA monomer) was added slowly. To maintain the pH value of the solution between 8.0–8.5, 0.2 M sodium hydroxide (NaOH) was added dropwise. After all the DMA was added, the reaction proceeded for 4 h, and then the product was dialyzed against sodium bicarbonate buffer (0.1 M, pH 8.5) for 2 days and lyophilized to harvest the DMA-modified co-polymer.
20, respectively). Co-polymerization was carried out in sealed ampoules with the protection of nitrogen at 50 °C for 24 h. The co-polymer was isolated from the polymerization mixture by precipitating into diethyl ether, purified by dialysis against distilled water and then freeze-dried. The HPMA–APMA co-polymer was dissolved in sodium bicarbonate buffer (0.1 M, pH 8.5) and DMA (3 molar equivalents to APMA monomer) was added slowly. To maintain the pH value of the solution between 8.0–8.5, 0.2 M sodium hydroxide (NaOH) was added dropwise. After all the DMA was added, the reaction proceeded for 4 h, and then the product was dialyzed against sodium bicarbonate buffer (0.1 M, pH 8.5) for 2 days and lyophilized to harvest the DMA-modified co-polymer.
For fluorescence resonance energy transfer (FRET) study, the RITC was conjugated to the HPMA polymers at a ratio of 2% (mol/mol).
The molecular weight (MW) and polydispersity index (PDI) of the obtained HPMA co-polymer were estimated by size exclusion chromatography on a Superose 200 10/300GL analytical column (Amersham Biosciences, NJ) calibrated with poly-HPMA fractions using a Fast Protein Liquid Chromatography (AKTA FPLC) system (Amersham Biosciences, NJ).32 The zeta potentials of various polymers were measured by using a Malvern Zetasizer NanoZS90 (Malvern Instruments Ltd, Malvern, UK).
2.3 Preparation of positively charged mesoporous silica nanoparticles
Firstly, we prepared the mesoporous silica nanoparticles as reported with some modifications.33 Briefly, the template CTAB (200 mg) was added into distilled water (100 mL), with vigorous stirring for 30 min at 70 °C. When a clear solution was obtained, 2 M NaOH (0.7 mL) was added into the solution for accelerating the speed of hydrolyzation and condensation of tetraethyl orthosilicate (TEOS). Then 0.9 mL TEOS was added followed by the addition of 0.09 mL 3-aminopropyltriethoxysilane (APTES). The mixture was stirred for 2 h. Finally, the mixture was washed with ethanol and purified water and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to obtain the positively charged mesoporous silica nanoparticles. To remove the excessive CTAB, the nanoparticles were suspended into a solution of ammonium nitrate (NH4NO3) in ethanol by refluxing twice for 2 h each time. Finally, the mixture were subjected to centrifugation at 14
000 rpm to obtain the positively charged mesoporous silica nanoparticles. To remove the excessive CTAB, the nanoparticles were suspended into a solution of ammonium nitrate (NH4NO3) in ethanol by refluxing twice for 2 h each time. Finally, the mixture were subjected to centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed with ethanol and water. The precipitate obtained was the positively charged amino-modified MSN (denoted as MSN).
000 rpm and washed with ethanol and water. The precipitate obtained was the positively charged amino-modified MSN (denoted as MSN).
To label the MSN core for further study, we synthesized FITC–APTES and Cy5–APTES formed by stirring FITC or Cy5 in ethanolic APTES solution in the dark for 24 h, respectively.34
2.4 Drug loading efficiency
For preparation of drug-loaded MSN (MSN–DTX), MSN (20 mg) was immersed into a 2 ml DTX solution (2 mg ml−1) for 24 h at room temperature. Excess reagent and drug were removed by washing with ethanol and water three times and then centrifugation to obtain MSN–DTX.To determine the drug loading capacity of nanoparticles, MSN–DTX was suspended in ethanol and ultrasonicated to release the drug. Subsequently, the suspension was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min. This process was repeated three times. The supernatant was harvested and analysed on a Dikma Diamonsil C18 column (250 × 4.6 mm, 5 mm) using an HPLC system (Agilent 1200 series, CA, USA). The mobile phase consisted of acetonitrile and double-distilled water (53/47, v/v) at a flow rate of 1.0 mL min−1 with a determination wavelength of 230 nm.
000 rpm for 20 min. This process was repeated three times. The supernatant was harvested and analysed on a Dikma Diamonsil C18 column (250 × 4.6 mm, 5 mm) using an HPLC system (Agilent 1200 series, CA, USA). The mobile phase consisted of acetonitrile and double-distilled water (53/47, v/v) at a flow rate of 1.0 mL min−1 with a determination wavelength of 230 nm.
2.5 Preparation of HPMA polymer-coated MSN hybrids
HPMA co-polymers R-P (HPMA–DMA) and I-P (HPMA–GG-OH) were dissolved at a concentration of 1.0 mg mL−1. Then, the solution of MSN–DTX (1 mg mL−1) was added dropwise to the above-mentioned solution under stirring. The mixture was stirred for another 20 min, and subsequently centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min to harvest the nanohybrid of HPMA co-polymer-coated MSNs (I-P@MSN–DTX, R-P@MSN–DTX).
000 rpm for 20 min to harvest the nanohybrid of HPMA co-polymer-coated MSNs (I-P@MSN–DTX, R-P@MSN–DTX).
2.6 Characterization of the MSN and HPMA polymer-coated MSNs
The mesostructure ordering of MSN was characterized by small-angle X-ray diffraction (SA-XRD) using Cu Kα radiation (40 kV and 100 mA). The DLS size and zeta potential of the MSN–DTX and HPMA co-polymer-coated MSNs were measured on a Zetasizer Nano ZS90 (Malvern Instruments, UK). The morphology and structure were observed via transmission electron microscopy (TEM).2.7 The assembly and disassembly of the nanohybrid
To determine the charge reversibility of the nanohybrid, I-P@MSN–DTX and R-P@MSN–DTX were suspended in PBS buffer solution (pH 7.4, 6.5 or 5.0). Then, all the samples were maintained in a shaker with a rate of 100 rpm at 37 °C. At predetermined time intervals, 200 μL sample was taken out and centrifuged (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min). Then the precipitate was suspended in an aqueous solution and the zeta potential was measured immediately.
000 rpm, 10 min). Then the precipitate was suspended in an aqueous solution and the zeta potential was measured immediately.
To further identify the coating and detachment effect of HPMA polymers, FRET experiment was executed. The MSN–DTX was labelled with FITC (MSN–DTX–FITC) and HPMA polymers were modified with RITC (RITC–I-P, RITC–R-P), which formed a FRET pair. The emission images of MSN–DTX–FITC with RITC–I-P and RITC–R-P were obtained, respectively. Then the RITC–I-P@MSN–DTX–FITC and RITC–R-P@MSN–DTX–FITC were loaded into a 96-well plate containing buffer solution with different pH values (pH 7.4, 6.5 or 5.0), and the samples were irradiated at a wavelength of 440 nm (ref. 35) and imaged with bandpass emission filters to obtain unmerged images of FITC fluorescence (520 nm) and RITC fluorescence (580 nm).
2.8 Pharmacokinetics study
To investigate the in vivo pharmacokinetics of MSN–DTX and nanohybrids, the Cy5-NHS was labelled onto the MSN–DTX. Then, healthy male BALB/c mice were randomly divided into four groups (n = 6) and intravenously injected with free Cy5, MSN–DTX–Cy5, I-P@MSN–DTX–Cy5, and R-P@MSN–DTX–Cy5 (equivalent of 0.1 μmol Cy5). At 5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h, blood samples (20 μL) were taken from the orbit, and the Cy5 intensity of each sample was measured by using a Varioskan Flash. The pharmacokinetic parameters were analysed using a noncompartmental model with PK Solver software.2.9 Cell experiments
For qualitative analysis, the cells were washed with PBS three times and fixed with fresh paraformaldehyde (4%) for 25 min, and stained with 4,6-diamidino-2-phenylindole (DAPI) for 5 min. Cell fluorescence images were obtained by confocal laser scanning microscopy (CLSM, Live 5 DUO, Carl Zeiss, Jena, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min to pellet the mitochondria. The precipitated mitochondria were collected and re-suspended in 0.3 mL PBS buffer (pH 7.4). Then the uptake by mitochondria (1 × 104) was measured with flow cytometry. Each assay was repeated in triplicate.
000g for 10 min to pellet the mitochondria. The precipitated mitochondria were collected and re-suspended in 0.3 mL PBS buffer (pH 7.4). Then the uptake by mitochondria (1 × 104) was measured with flow cytometry. Each assay was repeated in triplicate.
Simultaneously, the qualitative experiment was conducted. After incubation with samples, the cells were stained with Mitotracker Red (Invitrogen, USA). Then, the cells were washed with PBS three times and stained with 4,6-diamidino-2-phenylindole (DAPI) for 5 min. The images were obtained by CLSM (CLSM, Live 5 DUO, Carl Zeiss, Jena, Germany).
Changes in ROS production were monitored by measuring the oxidative conversion of cell permeable 2′,7′-dichlorofluorescein diacetate (DCFH-DA) to fluorescent dichlorofluorescein (DCF). Briefly, the HeLa cells were treated with MSN–DTX, I-P@MSN–DTX, and R-P@MSN–DTX at different pH values (pH 7.4 and 6.5) for 6 h. The cells were then harvested by centrifugation, washed twice with PBS, re-suspended in PBS, and incubated with 10 μM DCFH-DA for 30 min in the dark. The intracellular ROS levels were then examined immediately by measuring the fluorescence intensity of DCF using a Varioskan Flash.
2.10 In vivo biodistribution analysis
The in vivo biodistribution of different samples in tumors was measured by using a near-infrared reflection (NIR) fluorescence imaging system. Solid tumors were induced in nude mice by subcutaneously injecting the HeLa cells (1 × 107 cells per nude mouse) at the lateral aspect of the anterior limb. After achieving a volume of 400 mm3, free Cy5, Cy5-modified MSN–DTX–Cy5, I-P@MSN–DTX–Cy5, and R-P@MSN–DTX–Cy5 were administrated intravenously into tumor-bearing nude mice. At pre-determined time points (2 h, 8 h, and 24 h), the images were obtained. To compare the tumor distribution of the above samples, mice were sacrificed at 24 h post-injection. Tumors were dissected, washed with normal saline and imaged immediately using the in vivo imaging system. All animals used above received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals and all procedures were approved by Sichuan University Animal Care and Use Committee.2.11 In vivo antitumor efficacy
HeLa cells tumor-bearing nude mice were randomly divided into five groups (n = 5 per group). When the tumor volume reached about 100 mm3, the free DTX, MSN–DTX, I-P@MSN–DTX and R-P@MSN–DTX were administrated via a tail vein every two days four times at a dose of 5 mg kg−1 (equivalent of DTX) from the first day. Meanwhile, saline was used as a control group. Mice were weighed and the tumor width and length were measured every 2 days from day 1. The tumor size was calculated following the formula: (length × width2)/2. 21 days after treatment, mice were sacrificed and excised tumors were weighed. The tumor inhibitory rate (TIR) was calculated with the formula: TIR = [(WC − We)/WC] × 100%. Wc and We are the tumor weight of the control group and experimental group, respectively.2.12 Statistical analysis
Statistical significance in this study was analyzed by using the statistical package SPSS19.0. The quantitative data collected were expressed as mean ± S.D. Statistical significance was inferred at a value of p < 0.05.3. Results and discussion
3.1 Assembly and disassembly of the nanohybrid by the acid-stage pH stimulation
As we know, the β-carboxylic amide, which was developed by the amidation reaction between the DMA and amino groups on the HPMA co-polymers, could be stable at physiological pH and hydrolyzed when the pH value is below 6.8.25,28 On the other hand, HPMA co-polymers are considered to be excellent drug carriers due to their non-toxicity, non-immunogenicity, and biocompatibility.39 Hence, we could synthesize a DMA-modified HPMA (R-P) polymer integrating the superiorities (Fig. S1B†). To test our hypothesis, we synthesized another inert HPMA polymer (I-P, Fig. S1A†) without the pH-responsive ability. The molecular weights (MWs, Table S1†) of I-P and R-P were validated below the renal threshold (<45 kDa).40 Moreover, as the shielding materials, the zeta potentials (Table S1†) of the two polymers were demonstrated with negative charges as −22.4 mV and −17.5 mV, respectively.Serving as a positively charged core, the amino-decorated MSN was prepared. After subjecting MSNs to the TEM and DLS tests, the image of MSNs (Fig. S2A†) demonstrated that the size was around 100 nm, and the SA-XRD pattern (Fig. S2B†) with a single peak around 2° shows a partially ordered mesoporous distribution.41 Then we loaded DTX into the pores of MSNs to fabricate the MSN–DTX, and the drug loading capacity was 8.34%. After drug-loading, the TEM size of MSN–DTX barely increased (Fig. 1A), which was consistent with the change of the DLS size from 138 ± 3.7 nm to 140.4 ± 2.3 nm (Table 1). After being concealed by the negative HPMA polymers, the DLS size of I-P@MSN–DTX and R-P@MSN–DTX (Table 1) was remarkably increased to 184.5 ± 6.5 nm and 190.4 ± 4.9 nm, respectively. And the zeta potential of the nanohybrids dropped to a negative value of −21.3 mV and −18.2 mV from a positive charge (+27.3 mV). Besides, it could be found that a tethered co-polymer layer was around the MSN–DTX (pointed out by the arrows in Fig. 1A). All those phenomena indicated the successful coating of HPMA co-polymers on the MSN–DTX cores. Interestingly, there existed certain differences between the DLS sizes (Fig. S3†) and TEM sizes among all groups, the discrepancy was acceptable according to the previous reports, and the TEM size was closer to the true diameter due to the anhydrous condition of the MSN.41
| Samples | DLS size (nm) | Zeta potential (mV) | PDI |
|---|---|---|---|
| MSN | 138 ± 3.7 | +28.4 | 0.16 |
| MSN–DTX | 140.4 ± 2.3 | +27.3 | 0.18 |
| I-P@MSN–DTX | 184.5 ± 6.5 | −21.3 | 0.23 |
| R-P@MSN–DTX | 190.4 ± 4.9 | −18.2 | 0.22 |
Once amidated, the hydrolysing speed of the DMA amide bond was influenced by not only environmental pH but also the incubation duration. Due to the “pH ladder”, in which the pH values declined substantially from the physiological pH such as blood circulation and normal organs (pH 7.4) to the tumor microenvironment matrix (pH 6.0–7.0), then to endo/lysosomes (pH 4.0–5.0), the hybrids will go through a fluctuation of zeta potential as shown in Fig. 1B. When the charge-reversible hybrids travel in blood, the “core–shell” structure remains intact. As the hybrids leaked into the tumor extracellular matrix, the DMA amide bond became unstable and broken down by the mildly acidic pH; so the re-exposed positive charge of R-P and partial exposure of MSN cores are beneficial for quick cellular uptake. Upon being trapped into the endo/lysosome compartments, the final-stage pH-responsive variation follows, in which the hydrolyzing speed accelerates, coming with the strong repulsive interaction between the inside core and outside layer. Finally, the MSN core barely shows up.
To further confirm our supposition, we determined the charge-reversal ability of nanohybrids at different environmental pH values as a function of time. The pH values of 7.4, 6.5 and 5.0 stimulate the physiological pH, tumor extracellular matrix and endo/lysosome, respectively. As shown in Fig. 1C, the I-P@MSN–DTX showed a constant zeta potential at all pH values. However, when the pH values decreased from 7.4 to 6.5, the R-P@MSN–DTX suffered a charge conversion from −16.4 mV to +4.2 mV at first 2 h and reached a platform at 6 h due to the responsiveness of the DMA amide bond. While the pH value decreased to 5.0, the cleavage of DMA accelerated, and the R-P@MSN–DTX became positively charged even at the first 1 h. In a word, the DMA amide would be broken down faster at more acidic environment pH values, while maintaining the anionic status at normal tissue pH.
To further identify the interwork between the HPMA co-polymers and the MSN cores, we applied the FRET assay to detect the stability of hybrids at pH 7.4 and the process of disassembly at acidic pH over time. FITC was used as FRET donor, whereas RITC was labelled on the HPMA co-polymers as FRET receptor. Theoretically, FITC and RITC will generate the FRET effect when the two molecules are within 10 nm.42 Indeed, at pH 7.4, both RITC–I-P@FITC–MSN–DTX and RITC–R-P@FITC–MSN–DTX exhibit a prominent FRET signal as shown in Fig. S4A and B,† indicating energy transfer between FITC and RITC. This emerged FRET effect is also confirmed by the dual emission image of the two hybrids in Fig. 1D. Interestingly, when incubated at pH 6.5, the FITC fluorescence intensity of RITC–R-P@FITC–MSN–DTX increased whereas its RITC fluorescence intensity decreased, and the polarization change was rapid when incubated at a more acidic pH of 5.0. Obviously, the gradual separation of R-P and MSN–DTX should be responsible for the transformation. In contrast, the FRET signal was permanently visible during the whole 4 h for the RITC–I-P@FITC–MSN–DTX in all tests. Collectively, the assembly of the nanohybrids could shield the positive charges of the MSN cores thus maintaining the system intact at physiological pH, but when going through an acidic environment, the DMA amide bond would be degraded in a multi-stage procedure caused by the combined influencing factors of the acidic stage together with incubation periods.
To further study the influence of the pH value upon the drug release of formulations, we investigated the drug release profiles at pH 7.4, 6.5 and 5.0. The DTX loaded MSN–DTX, I-P@MSN–DTX and R-P@MSN–DTX were incubated at different pH values. As shown in Fig. 1E, the nude MSN–DTX core showed a pH-dependent drug release feature because the decreased pH impacted upon the hydrogen bonds or polar interactions between DTX and the MSN.43 Meanwhile, about 32.5% of DTX was released from MSN–DTX at pH 7.4 over 48 h, while less than 20% in the groups of I-P@MSN–DTX and R-P@MSN–DTX. This indicated that the coating of polymers would slow down the speed of drug leakage at normal tissue pH. In sharp contrast, when R-P@MSN–DTX was incubated at pH 6.5 and 5.0, about 23.5% and 33% of DTX were released in the first 4 h due to the pH-responsive ability of R-P. Interestingly, even at pH 5.0, the drug release rate of R-P@MSN–DTX was detained compared to MSN–DTX. It is likely that the hydrolysis speed of amides on the R-P would be responsible for the interruptive drug release, which might protect the DTX from the acidic endo/lysosomes. Collectively, the R-P coated on the MSN–DTX has the ability of controlling drug release due to the multistage pH-responsiveness consistent with the charge-reversible study.
3.2 Enhanced persistence in blood circulation and tumor accumulation with initial pH-responsiveness
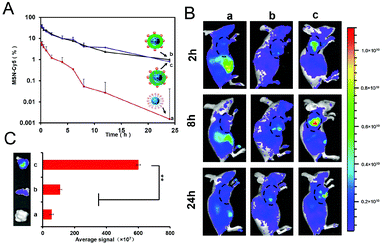
As we know, the blood circulation time of the anionic materials is relatively longer, resulting in the EPR effect and promoted tumor accumulation, which is a guarantee of satisfactory cell uptake and anti-tumor efficiency. As mentioned above, the coating of HPMA co-polymers would ensure that the core–shell structure is intact at physiological pH due to the negative surface charge.To investigate the in vivo circulation behaviour of the core–shell structure, the pharmacokinetics profiles of the formulations were studied for 24 h. As shown in Fig. 2A, the bare MSN–Cy5 demonstrated quick blood circulation clearance with a shorter half-life period (t1/2) of 2.89 h, which may be due to the positive charge. In contrast, the co-polymer-shielding hybrids I-P@MSN–DTX–Cy5 and R-P@MSN–DTX–Cy5 possessed a longer blood circulation persistence with t1/2 values of 5.59 h and 5.32 h, respectively, which are 1.93-fold and 1.84-fold compared to the MSN core. In a word, the distinct in vivo fate of coated and uncoated cores would be inevitable in the case of serum inhibition and entanglement with opsonin, and the prolonged circulation time was achieved by the coating of HPMA co-polymers.
Thanks to the extended circulation time in blood, considerable amounts of nanohybrids will extravagate into the tumor extracellular matrix. To explore the tumor accumulation, we further conducted a living image study at 2 h, 8 h, and 24 h. As shown in Fig. 2B, in agreement with the pharmacokinetics study, the bare MSN scarcely remained in tumors due to the quick circulation clearance. In sharp contrast, the concealed groups possessed better accumulation, which peaked around 8 h. More interestingly, R-P@MSN–DTX–Cy5 showed a widespread fluorescence area and stronger intensity compared to I-P@MSN–DTX–Cy5, which may be caused by the first-step pH responsiveness. Finally, further insight into the isolated tumor tissues at 24 h was obtained. Consistent with the living image study, R-P@MSN–DTX–Cy5 showed a strong fluorescence intensity among all groups after 24 h, which is 5.49-fold and 10.7-fold that of the I-P coated MSN and bare MSN, respectively. Notably, the protection of co-polymers will delay the clearance of the drug-loaded MSN core, further promoting the EPR effect. Moreover, the first-stage charge reversal caused by the hydrolyzation of the DMA amide contributed to the abundant tumor tissues accumulation.
3.3 Characterization of endocytosis and endo/lysosome escape
Once trapped around the tumor tissues, quick endocytosis occurred. As displayed in Fig. 3A, the cell uptake of the formulations occurred in a time-dependent manner. Moreover, owing to the first-stage pH responsiveness of R-P, the R-P@MSN–DTX showed enhanced cell uptake at pH 6.5 compared to that at pH 7.4, which is 1.3-fold higher at 2 h and 1.9-fold at 4 h, respectively (p < 0.05). Consistent with the quantitative analysis, the exploration of confocal laser scanning microscopy (CLSM) presented an increased green fluorescence intensity of R-P@MSN–DTX in different incubation situations at 4 h. However, I-P@MSN–DTX exhibited a fair amount of fluorescence both in 2 h and 4 h, identified by the quantitative and qualitative analyses. The data presented above showed that cell uptake was influenced by the pH of incubation solution, which stimulated the physiological condition and tumor extracellular matrix. The discrepancy was derived from the gradually emerging positive charge of charge reversal, which pulled the cationic hybrids and the anionic cell membrane closer through electrostatic interaction. As expected, the bare MSN possessed maximum cell uptake due to the strong positive charge.After being internalized into cells, it is inevitable for nanohybrid to be sealed into endo/lysosomes. In order to achieve subcellular targeting, effective endo/lysosome escape is essential. So, we next determined the endo/lysosome escape ability of the nanohybrids. As shown in Fig. 3C, the escape for I-P@MSN–DTX is unsuccessful compared to the other groups, whose Pearson correlation coefficient (Rr) is beyond 0.5.44 Moreover, the escape ability of R-P@MSN–DTX and MSN–DTX is comparative according to the co-localization area. It was reported that the cationic MSN could escape from the endo/lysosome with the help of positive groups such as amino45 and modified surfaces. When the nanohybrids enter into the endo/lysosome, I-P-coated MSN could not be liberated due to the integrated structure defended by the pH-insensitive I-P. Then the cationic amino group was concealed totally, and failed to trigger endo/lysosomal escape through the proton sponge effect. On the contrary, affected by the more acidic lysosomal environment, the hydrolysis of DMA amide was accelerated according to the prior study, thus the MSN core detached itself from the coating layer because of the powerful repelling force between positive cores and hydrolyzed cationic HPMA co-polymers. Eventually, with the assistance of the amino group decorated on the surface of MSN, the MSN core successfully escaped from the endo/lysosome.
3.4 Subcellular fate of nanohybrids
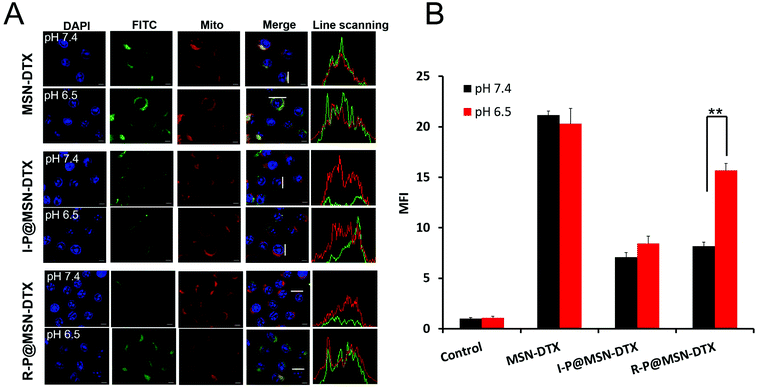
As reported, the majority of the positively charged substance is able to accumulate around negative mitochondria.46 To further demonstrate the subcellular distribution, we executed the CLSM analysis for the formulations after 6 h incubation at different pH values. Once escaped from the endo/lysosome, the bare MSN core will directly head to the mitochondria. As shown in Fig. 4A, the overlapping area appearing in the R-P@MSN–DTX at pH 7.4 and 6.5 is discrepant, identified by the FCM analysis displayed in Fig. 4B. The mitochondrial uptake of R-P@MSN–DTX at pH 6.5 is 1.8 times that of pH 7.4 (p < 0.05). In contrast, there is no noticeable mitochondrial accumulation of I-P@MSN–DTX because of the merely yellow fluorescence intensity. The determined mitochondriotropics of positive cores was consistent with a previous report.14 Altogether, the second-stage pH-responsiveness of R-P would promote the mitochondrial accumulation as a consequence of the accelerated hydrolysis of DMA amide in more acidic endo/lysosomes. And interestingly, the mitochondrial accumulation was a combination of increased cellular uptake and mitochondrial targeting due to the electrostatic interaction induced by the multistep re-exposed positive MSN core.3.5 Specific mitochondrial damage after subcellular targeting
Once stimulated by the DTX released from the targeted MSN–DTX, the mitochondria would present a series of reactions. We first test the accommodation ability of mitochondria for reactive oxygen species (ROS). We applied DCFH-DA to visualize the elevation of the ROS level. In the normal status, the DCFH-DA travels easily across the cell membrane with no significant fluorescence intensity, while oxidated by the ROS in the cells, the DCFH-DA will transform into DCF accompanied by detectable green fluorescence intensity. In Fig. 5A, there is no visible green fluorescence at both pH 7.4 and 6.5 after treating with I-P@MSN–DTX. In contrast, a significant green fluorescence emerged at pH 6.5 after incubation with R-P@MSN–DTX compared with pH 7.4. Consistent with the CLSM images, the quantitative analysis shows that the ROS level of R-P@MSN–DTX at pH 6.5 increased 1.31-fold and 2.82-fold compared to the R-P@MSN–DTX at pH 7.4 and the control group. Therefore, it is not surprising that MSN–DTX possesses the highest ROS level and collapse of Δψm occurs due to maximum cell uptake and mitochondrial accumulation.The release of ROS is accompanied by mitochondrial depolarization, which is a response to drug stimuli. At a promoted ROS level, the permeability transition pore complex (PTPC) on the mitochondrial membrane assumes a high-conductance state to allow the entry of small solutes into the mitochondrial matrix, which results in the collapse of Δψm.47 So, it is reasonable that the increased ROS level is accompanied by the dissipation of the mitochondrial membrane potential. Then we next measure the mitochondrial membrane potential of each formulation. A MitoProbe JC-1 assay kit was used to assess Δψm. JC-1(red) aggregates in normal mitochondria, and the color changes from red to green when the membrane potential collapses. A decreased fluorescence ratio is consistent with the collapse of Δψm.38 As shown in Fig. 5D, the collapse of Δψm about R-P@MSN–DTX at pH 7.4 was 1.93-times that of pH 6.5, which is consistent with Fig. 5C. In Fig. 5C, the green fluorescence of R-P@MSN–DTX at pH 6.5 was stronger compared to that at pH 7.4. Moreover, the elevated cellular uptake caused by the first-stage pH-responsive reversal of R-P and increased mitochondrial targeting induced by the re-exposure of the core will take the responsibility for the variation of mitochondrial features.
3.6 pH-Dependent cytotoxicity analysis
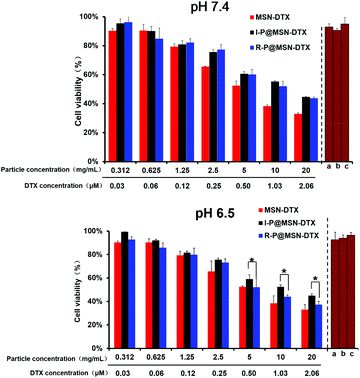
To examine the cytotoxicity induced by the nanohybrids at different environmental pH values, the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was applied. As shown in Fig. 6, both at pH 7.4 and 6.5, the drug-unloading carriers exhibited no significant cytotoxicity at a higher particle concentration compared to the highest drug-loading particle concentration, which confirmed the excellent performance of HPMA co-polymers and MSN acts as a vector. Then, every drug-loading therapeutic at pH 7.4 revealed a dose-dependent characteristic, and the two hybrids I-P@MSN–DTX and R-P@MSN–DTX possessed a similar cytotoxicity, with an IC50 of 1.13 μM and 1.21 μM (DTX concentration), respectively. Moreover, with the acidic pH simulating the tumor microenvironment, the R-P@MSN–DTX exhibited significantly higher cytotoxicity with an IC50 of 0.85 μM compared to pH 7.4 (p < 0.05), suggesting the advantage of pH-responsiveness for the first-step charge-reversal to enhance endocytosis and for the second-step with entire exposure of the positive core for mitochondrial targeting. Meanwhile, we determined the cytotoxicity of free DTX. As shown in Fig. S5,† the IC50 of DTX at pH 7.4 and 6.5 is 0.82 μM and 0.76 μM, respectively. The cell suppression of free DTX was stronger than that of other tested nanohybrids because of easier diffusion of free drugs.26 In addition, the bare positive MSN showed the highest cytotoxicity at both pH 7.4 and 6.5, with an IC50 of 0.70 μM and 0.67 μM, respectively, which further certified that a significant antitumor efficacy is related to the uncovered positive charge.3.7 In vivo antitumor ability
Inspired by the in vitro experiments, the in vivo pharmacodynamic effect was studied on HeLa tumor-bearing nude mice. When the tumor volume is around 100 mm3, the nude mice were injected through a tail vein at a dose of 5 mg kg−1 mouse (equivalent of DTX) for different formulations at 0th, 3rd, 6th, and 9th day. As shown in Fig. 7A, the tumor of saline treated mice grew rapidly, while the growth of the tumors was suppressed by free DTX or DTX-loading formulations. Apparently, in Fig. 7C, the mice treated with R-P@MSN–DTX achieved the highest tumor inhibition (72.6%), which was 1.16-times and 2.25-times that of free DTX (62.3%) and I-P@MSN–DTX (32.2%), respectively. Interestingly, compared to the best result achieved by MSN–DTX in vitro such as mitochondrial targeting and cytotoxicity, the in vivo study was a little worse than R-P@MSN–DTX with the suppression rate of 66.9% (p < 0.05). Finally, to evaluate the safety of formulations, we adopt the body weight as an assessment criterion. As shown in Fig. 7D, all groups had no obvious changes except for MSN–DTX, which confirmed its good antitumor ability accompanied by serious side effects proved by the rapid descending of the body weight curve (p < 0.05 vs. saline). In a word, the reversible ending of the in vivo study about R-P@MSN–DTX should be attributed to the ability of the R-P of concealing positive charge for extended blood circulation time and multistage exposure of the MSN–DTX core for cytotoxicity, and with no significant side effects.4. Conclusion
In summary, a novel pH-responsive charge-reversible MSN based nanohybrid was constructed for mitochondrial targeting. Under the shielding of HPMA polymers, the nanohybrid possessed a maximal 1.93-fold prolonged t1/2 compared to the bare MSNs, and gave a better EPR effect. Then, a co-localization study of MSN and mitochondria and quantitative analysis for the isolated mitochondrial certified its good mitochondrial targeting. Furthermore, the pH-stimulated R-P@MSN–DTX exhibited promoted cytotoxicity in HeLa cells. This result is likely attributed to the promoted endocytosis and successful mitochondrial targeting. Eventually, an in vivo study was conducted on HeLa tumor-bearing nude mice and a peak tumor inhibition rate of 72.6% was obtained, as a result of this nanohybrid with a comprehensive superiority of “assembly–disassembly”, that is assembly at normal tissue pH for a prolonged blood circulation time and gradual disassembly with the decreasing pH for cell internalization and mitochondrial targeting. In conclusion, this research demonstrated the strategy of applying a disassembled pH-responsive nanohybrid for anti-tumor therapy both in vitro and in vivo.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (81473167) and the Doctoral Fund of the Ministry of Education of China (2013018111001).Notes and references
- S. Fulda, L. Galluzzi and G. Kroemer, Nat. Rev. Drug Discovery, 2010, 9, 447–464 CrossRef CAS PubMed.
- P. Lu, B. J. Bruno, M. Rabenau and C. S. Lim, J. Controlled Release, 2015, 240, 38–51 CrossRef PubMed.
- L. Milane, M. Trivedi, A. Singh, M. Talekar and M. Amiji, J. Controlled Release, 2015, 207, 40–58 CrossRef CAS PubMed.
- J. W. Simpkins, J. Wang, X. Wang, E. Perez, L. Prokai and J. A. Dykens, Curr. Drug Targets: CNS Neurol. Disord., 2005, 4, 69–83 CrossRef CAS.
- P. A. Kiberstis, Science, 2004, 303, 1439–1439 CrossRef.
- V. B. Ritov, E. V. Menshikova, K. Azuma, R. Wood, F. G. Toledo, B. H. Goodpaster, N. B. Ruderman and D. E. Kelley, Am. J. Physiol.: Endocrinol. Metab., 2010, 298, E49–E58 CrossRef CAS PubMed.
- D. C. Wallace, Nat. Rev. Cancer, 2012, 12, 685–698 CrossRef CAS PubMed.
- G. Kroemer, L. Galluzzi and C. Brenner, Physiol. Rev., 2007, 87, 99–163 CrossRef CAS PubMed.
- Z. Chen, L. Zhang, Y. Song, J. He, L. Wu, C. Zhao, Y. Xiao, W. Li, B. Cai and H. Cheng, Biomaterials, 2015, 52, 240–250 CrossRef CAS PubMed.
- L.-F. Dong, V. J. Jameson, D. Tilly, L. Prochazka, J. Rohlena, K. Valis, J. Truksa, R. Zobalova, E. Mahdavian and K. Kluckova, Free Radicals Biol. Med., 2011, 50, 1546–1555 CrossRef CAS PubMed.
- M. Han, M. R. Vakili, H. Soleymani Abyaneh, O. Molavi, R. Lai and A. Lavasanifar, Mol. Pharm., 2014, 11, 2640–2649 CrossRef CAS PubMed.
- M. Ross, G. Kelso, F. Blaikie, A. James, H. Cocheme, A. Filipovska, T. Da Ros, T. Hurd, R. Smith and M. Murphy, Biochemistry, 2005, 70, 222–230 CAS.
- R. A. Smith, R. C. Hartley and M. P. Murphy, Antioxid. Redox Signaling, 2011, 15, 3021–3038 CrossRef CAS PubMed.
- R. Mo, Q. Sun, J. Xue, N. Li, W. Li, C. Zhang and Q. Ping, Adv. Mater., 2012, 24, 3659–3665 CrossRef CAS PubMed.
- J. Callahan and J. Kopecek, Biomacromolecules, 2006, 7, 2347–2356 CrossRef CAS PubMed.
- V. Cuchelkar, P. Kopečková and J. Kopecek, Mol. Pharm., 2008, 5, 776–786 CrossRef CAS PubMed.
- S. Biswas, N. S. Dodwadkar, P. P. Deshpande and V. P. Torchilin, J. Controlled Release, 2012, 159, 393–402 CrossRef CAS PubMed.
- L. Jiang, L. Li, X. He, Q. Yi, B. He, J. Cao, W. Pan and Z. Gu, Biomaterials, 2015, 52, 126–139 CrossRef CAS PubMed.
- D. Y. Cho, H. Cho, K. Kwon, M. Yu, E. Lee, K. M. Huh, D. H. Lee and H. C. Kang, Adv. Funct. Mater., 2015, 25, 5479–5491 CrossRef CAS.
- J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Y. Lin, Small, 2010, 6, 1952–1967 CrossRef CAS PubMed.
- F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang and T. Jiang, Nanomedicine, 2015, 11, 313–327 CAS.
- J. S. Souris, C.-H. Lee, S.-H. Cheng, C.-T. Chen, C.-S. Yang, A. H. Ja-an, C.-Y. Mou and L.-W. Lo, Biomaterials, 2010, 31, 5564–5574 CrossRef CAS PubMed.
- J. Zhong, L. Li, X. Zhu, S. Guan, Q. Yang, Z. Zhou, Z. Zhang and Y. Huang, Biomaterials, 2015, 65, 43–55 CrossRef CAS PubMed.
- L. Li, Q. Yang, Z. Zhou, J. Zhong and Y. Huang, Biomaterials, 2014, 35, 5171–5187 CrossRef CAS PubMed.
- Q. Yang, L. Li, W. Sun, Z. Zhou and Y. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 13251–13261 CAS.
- M. Talelli, C. Rijcken, C. Van Nostrum, G. Storm and W. Hennink, Adv. Drug Delivery Rev., 2010, 62, 231–239 CrossRef CAS PubMed.
- L. Li, W. Sun, J. Zhong, Q. Yang, X. Zhu, Z. Zhou, Z. Zhang and Y. Huang, Adv. Funct. Mater., 2015, 25, 4101–4113 CrossRef CAS.
- M.-C. Bissery, D. Guénard, F. Guéritte-Voegelein and F. Lavelle, Cancer Res., 1991, 51, 4845–4852 CAS.
- V. Omelyanenko, P. Kopečková, C. Gentry and J. Kopeček, J. Controlled Release, 1998, 53, 25–37 CrossRef CAS PubMed.
- Q. Yang, L. Li, X. Zhu, W. Sun, Z. Zhou and Y. Huang, RSC Adv., 2015, 5, 14858–14870 RSC.
- Z. Zhou, L. Li, Y. Yang, X. Xu and Y. Huang, Biomaterials, 2014, 35, 6622–6635 CrossRef CAS PubMed.
- J. Lu, M. Liong, S. Sherman, T. Xia, M. Kovochich, A. E. Nel, J. I. Zink and F. Tamanoi, Nanobiotechnology, 2007, 3, 89–95 CrossRef CAS PubMed.
- X. Huang, L. Li, T. Liu, N. Hao, H. Liu, D. Chen and F. Tang, ACS Nano, 2011, 5, 5390–5399 CrossRef CAS PubMed.
- W. Shan, X. Zhu, M. Liu, L. Li, J. Zhong, W. Sun, Z. Zhang and Y. Huang, ACS Nano, 2015, 9, 2345–2356 CrossRef CAS PubMed.
- A. Banerjee, J. Qi, R. Gogoi, J. Wong and S. Mitragotri, J. Controlled Release, 2016, 238, 176–185 CrossRef CAS PubMed.
- L. Jiang, L. Li, X. He, Q. Yi, B. He, J. Cao, W. Pan and Z. Gu, Biomaterials, 2015, 52, 126–139 CrossRef CAS PubMed.
- W. Sun, L. Li, Q. Yang, W. Shan, Z. Zhang and Y. Huang, Mol. Pharm., 2015, 12, 4124–4136 CrossRef CAS PubMed.
- Q. Yang, Y. Yang, L. Li, W. Sun, X. Zhu and Y. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 6661–6673 CAS.
- R. Zhang, J. Yang, D. C. Radford, Y. Fang and J. Kopeček, Macromol. Biosci., 2016 DOI:10.1002/mabi.201600125.
- Q. He, Y. Gao, L. Zhang, Z. Zhang, F. Gao, X. Ji, Y. Li and J. Shi, Biomaterials, 2011, 32, 7711–7720 CrossRef CAS PubMed.
- J. Xu, J. Liang, J. Li and W. Yang, Langmuir, 2010, 26, 15722–15725 CrossRef CAS PubMed.
- Q. Liu, J. Zhang, W. Sun, Q. R. Xie, W. Xia and H. Gu, Int. J. Nanomed., 2012, 7, 999–1013 CAS.
- V. Zinchuk and O. Grossenbacher-Zinchuk, Prog. Histochem. Cytochem., 2009, 44, 125–172 CrossRef CAS PubMed.
- D.-M. Huang, Y. Hung, B.-S. Ko, S.-C. Hsu, W.-H. Chen, C.-L. Chien, C.-P. Tsai, C.-T. Kuo, J.-C. Kang and C.-S. Yang, FASEB J., 2005, 19, 2014–2016 CrossRef CAS PubMed.
- M. P. Murphy and R. A. Smith, Adv. Drug Delivery Rev., 2000, 41, 235–250 CrossRef CAS PubMed.
- N. R. Brady, A. Hamacher-Brady, H. V. Westerhoff and R. A. Gottlieb, Antioxid. Redox Signaling, 2006, 8, 1651–1665 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr07004c |
| This journal is © The Royal Society of Chemistry 2017 |