Depositing CdS nanoclusters on carbon-modified NaYF4:Yb,Tm upconversion nanocrystals for NIR-light enhanced photocatalysis†
Meijie
Tou‡
,
Yuanyuan
Mei‡
,
Song
Bai
*,
Zhenguo
Luo
,
Yong
Zhang
and
Zhengquan
Li
*
Institute of Physical Chemistry and Department of Chemistry, Zhejiang Normal University, Jinhua, Zhejiang 321004, P. R. China. E-mail: zqli@zjnu.edu.cn; songbai@zjnu.edu.cn
First published on 19th November 2015
Abstract
High-quality hexagonal NaYF4:Yb,Tm upconversion nanocrystals (UCNs) prepared in organic solutions display uniform sizes and strong UC emissions, but they possess a hydrophobic surface which hinders combining them with various semiconductor nanocrystals (NCs) to form a hybrid NIR-activated photocatalyst. Herein we present a facile approach to modify hydrophobic UCNs with a uniform carbon layer and enable them with hydrophilicity and surface functionalization. The carbon shell provides a good substrate for enriching with metal ions and in situ generation of CdS nanoclusters on the particle surface which can utilize both the upconverted UV and visible emissions. The developed NaYF4:Yb,Tm@C@CdS nanoparticles are characterized with TEM, SEM, XRD, PL and UV-Vis spectra and their formation mechanism is elucidated. The products display good photocatalytic activity under visible light and obviously enhanced performance under Vis-NIR light, due to the efficient utilization of UC emissions and the strong adsorption capacity of the carbon shell. The working mechanism of the hybrid photocatalysts is also proposed.
1. Introduction
Environmental pollution and energy shortage are both global crises and threats to the long-term development of human society.1–3 Among potential solutions, semiconductor photocatalysis utilizing solar energy as an environmental remedy is considered an economic, renewable, clean and safe technology.4–6 However, one big limitation of semiconductor photocatalysis is the narrow absorption range of semiconductors which can only make use of ultraviolet (UV) or visible (Vis) light. Although many strategies have been employed to broaden or extend the absorption range of semiconductors such as doping, dye sensitization, and coupling with noble metals or other semiconductors, the main absorption of most semiconductors is still limited to the UV or Vis region.7–10 Utilization of solar energy in the near-infrared (NIR) region is still rarely explored while NIR light comprises ca. 48% of the solar spectrum.11–13 To overcome this limitation, the creation of hybrid photocatalysts with upconversion nanocrystals (UCNs) as light converters has been regarded as one of the promising solutions.14,15 With the capability of the NIR photons to be upconverted to UV and/or Vis photons, the UCNs can absorb NIR light and activate the semiconductors for photocatalytic applications. Development of uniform and efficient UCN–semiconductor hybrid nanostructures is thus of great importance to achieve such kinds of NIR-activated photocatalysts.In a hybrid NIR-activated photocatalyst, the activation ability for the semiconductor relies on the UC emission of the UCNs. Thus, the overlap of the emission and absorption spectra between the UCNs and the semiconductors is a very important parameter to determine their photocatalytic efficiency. Nowadays, hexagonal-phase (β-) NaYF4:Yb,Tm NCs have been proven to be one of the best UC phosphors and the combination of NaYF4:Yb,Tm NCs with TiO2 or ZnO shells has been extensively explored.16–18 However, it is known that NaYF4:Yb,Tm NCs exhibit strong blue emissions and relatively low UV emissions, while the TiO2 and ZnO shells can only harness the UV emissions. For efficient utilization of the upconverted Vis emissions, the combination of NaYF4:Yb,Tm NCs with a narrow band gap semiconductor such as CdS should be a better choice.19 But, different from the synthesis of the TiO2 shell which can be achieved via a traditional sol–gel process, the preparation of uniform core–shell structured UCNs with other semiconductors is still challenging, due to the difference in growing habits and/or conditions for different NCs. Recently, Yu et al. attempted the synthesis of a hybrid photocatalyst consisting of NaYF4:Yb,Tm microrods and CdS NCs.20 The synthesis involved the individual preparation of NaYF4:Yb,Tm and CdS particles, functionalizing them with different groups, and then linking them through molecular chains. Such a process is very tedious and the developed NaYF4:Yb,Tm/CdS photocatalysts exhibit a large particle size, low UC fluorescence, and easy aggregation in solution. Furthermore, the stability of such hybrid particles is also problematic because of the low thermal stability of the molecular chains and the high mobility of the solid NCs in solution. It is highly desirable to develop convenient and reliable approaches to synthesize hybrid NIR-activated photocatalysts with high uniformity, strong UC emissions, excellent dispersibility, and high structural stability.
In contrast to the UCNs prepared under hydrothermal conditions, the UCNs obtained from high-temperature organic solutions are more uniform in size and have stronger UC fluorescence.21,22 However, a big limitation of these high-quality UCNs is their hydrophobic surface, which hinders their dispersion in aqueous solution. On the other hand, to avoid the tedious process of surface modification and molecular linking of two types of NCs, direct deposition of the semiconductor NCs on the UCNs is preferred. But this synthetic strategy requires a hydrophilic, functional and active surface on the UCNs as a substrate. Carbon spheres composed of carbonous polymer possess a hydrophilic and flexible surface with many functional groups, which are easily suspended in aqueous solution and have been widely used as templates to deposit various oxide and sulfide semiconductor NCs.23,24 If a carbon shell could be made to cover the hydrophobic UCNs, these carbon-coated UCNs would be hydrophilic and build a versatile surface for the hybridization of various semiconductor NCs. Furthermore, a carbon shell can efficiently absorb and enrich the polluted species around the hybrid photocatalysts, which can also greatly enhance the photocatalytic effect.25–27 Moreover, some of the literature has also suggested that carbon shells can harvest some Vis light and facilitate the electron–hole separation for the photocatalysts.28,29 These features may also benefit the overall photocatalytic efficiency of the hybrid NIR-activated photocatalysts.
In this work, we present a facile route to formulate hybrid NIR-activated photocatalysts by coating a uniform carbon shell on the hydrophobic UCNs and then directly depositing semiconductor NCs on their surface. As a proof-of-concept work, we employed uniform β-NaYF4:Yb,Tm nanoplates as the UCNs and CdS nanoclusters as the semiconductor NCs. Specifically, we developed a reverse micelle approach to coat a uniform carbon shell on the NaYF4:Yb,Tm nanoplates though carbonization of glucose. The carbon shell can efficiently enrich the metal ions and serve as a powerful substrate for directly generating CdS NCs on the NaYF4:Yb,Tm nanoplates. The developed core–shell NaYF4:Yb,Tm@C@CdS NPs exhibit strong UC emissions, uniform sizes, and strong capability for adsorbing the polluted species. This hybrid photocatalyst can be directly used for the degradation of various dye pollutants under Vis light, and exhibits enhanced activity under Vis-NIR light. This work may shed some new light on the design and synthesis of hybrid photocatalysts and their environmental applications.
2. Experimental
2.1 Preparation of β-NaYF4:Yb(20%),Tm(0.5%) NCs
Monodisperse β-NaYF4:Yb,Tm NCs were prepared with a user-friendly protocol we previously developed.30 All the chemical reagents were used as received without further purification. At the start, 0.8 mmol YCl3, 0.2 mmol YbCl3, and 0.05 mmol TmCl3 were mixed with 4 mL oleic acid and 16 mL octadecene (ODE) in a 50 mL three-neck flask. The solution was heated to 160 °C to form a yellow transparent solution and then cooled down to room-temperature. Subsequently, 10 mL of a methanol solution containing 2.5 mmol NaOH and 4 mmol NH4F was added into the flask and stirred for 30 min. After that, the solution was slowly heated to remove methanol and degassed at 100 °C for 20 min. The flask was heated to 300 °C and maintained at this temperature for 1.5 h under Ar protection. After naturally cooling down to room-temperature, the products were precipitated from the solution by ethanol and centrifuged out with a speed of 5000 rpm. The products were washed with cyclohexane and ethanol four times, and finally dispersed in cyclohexane with a concentration of around 0.1 M.2.2 Coating a carbon shell on β-NaYF4:Yb,Tm NCs
The above β-NaYF4:Yb,Tm NCs were initially modified with surfactant to become hydrophilic and then used as seeds to coat them with a carbon shell using a hydrothermal approach. In a typical synthesis, 10 mL deionized (DI) water and 0.05 g cetyltrimethyl ammonium bromide (CTAB) were mixed in a 25 mL flask and heated to 40 °C for 10 min for fast dissolution of the surfactant. Then, 0.02 mmol of NaYF4:Yb,Tm NCs in cyclohexane solution was added to the above solution and the temperature was raised to 78 °C to slowly evaporate the cyclohexane. After a transparent solution was formed, the solution was transferred into a 50 mL autoclave. Following the addition of 0.4 g glucose to it, the autoclave was heated to 160 °C and maintained at this temperature for 4 h. After the autoclave was cooled down to room-temperature, the products were centrifuged off from the solution and washed with ethanol and DI water, and finally dispersed in DI water for the next step.2.3 Deposition of CdS nanoclusters on NaYF4:Yb,Tm@C NPs
In a typical synthesis, 15 mL DI water and 0.02 mmol NaYF4:Yb,Tm@C NPs were mixed in a 25 mL flask. The solution was heated to 80 °C, following the addition of 0.5 g polyvinylpyrrolidone (PVP) and 0.026 g Cd(NO3)2·4H2O to it. The solution was then kept under stirring for 30 min for the adsorption of Cd2+ ions on the carbonous shell of the NaYF4:Yb,Tm@C NPs. Subsequently, 0.026 g of thioacetamide (TAA) was added to the solution and the solution was stirred at 80 °C for 1 h. The flask was then equipped with a condenser and put in a microwave irradiator (MCR-3E, 280W) and irradiated for 10 min. After the microwave irradiator was turned off, the products were centrifuged off from the flask and washed with DI water and ethanol thrice. Finally, the products were dried under vacuum at 60 °C for 4 h.2.4 Characterization
Powder X-ray diffraction (XRD) was carried out on a Philips X'Pert Pro X-ray diffractometer equipped with Cu Kα radiation. Transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDS) were performed on a JEOL 2010F TEM operated at 300 kV. The TEM samples were prepared by dropping a suspension of NPs on to a copper grid. Scanning electron microscopy (SEM) was performed with a Hitachi S-4800 field emission SEM with an accelerating voltage of 15 kV. Fluorescence spectra were acquired on a Hitachi F-7000 spectrometer equipped with a commercial 980 nm NIR laser. UV-Vis absorption spectra were obtained on a Shimazhu UV-2450 UV-Vis spectrometer. X-ray photoelectron spectroscopy (XPS) measurements were done on a ESCALAB MKII XPS system with a Mg Kα source and a charge neutralizer.2.5 Photocatalytic measurements
Photocatalytic activities of the β-NaYF4:Yb,Tm@C@CdS NPs were evaluated by degradation of rhodamine B (RhB) and methylene blue (MB) solutions under the irradiation of a Xe lamp (50 W). Different irradiation bands were obtained through rationally choosing the following filters: Vis bandpass (400–780 nm) and NIR bandpass (780–2500 nm). In a typical process, 10 mg of the as-prepared products was put into 50 mL of RhB solution or MB solution (5 × 10−5 M) in a beaker. The solution was then stirred for 12 h in the dark to reach an adsorption–desorption equilibrium between the NPs and the dye molecules. Subsequently, the beaker was exposed to the irradiation of the Xe lamp accompanied with suitable filters. Aliquots were intermittently collected at given time intervals to measure the concentration of RhB or MB by UV-Vis spectroscopy.3. Results and discussion
3.1 Synthetic strategy and formation mechanism
The synthetic process from primitive NaYF4:Yb,Tm NCs to double shell-coated NaYF4:Yb,Tm@C@CdS NPs is illustrated in Scheme 1. Since the prepared NaYF4:Yb,Tm NCs have a hydrophobic surface due to the surface-adsorbed oleic acid (OA), they are unable to be dispersed in water. However, the process of hydrothermal carbonization of glucose is generally performed in aqueous solution. To modify the particles’ surface, we employed a reverse micelle method to attach a monolayer of surfactants on these NCs (see Fig. S1†). The hydrophobic tails of the surfactants can bind oleic acids on the NCs’ surface through van der Waals interactions, leaving their hydrophilic heads pointing outward. After modification with surfactants, the NaYF4:Yb,Tm NCs can be well dispersed in water and mixed with glucose solution. When the mixed solution was hydrothermally treated in an autoclave for 4 h, a layer of carbonous polymer was created on each NaYF4:Yb,Tm particle. Note that the carbonous polymer shell consists of crosslinked aromatic compounds and lots of functional groups such as –OH, –CHO, and –COOH.31 As such, the carbon shell can efficiently adsorb Cd2+ ions around the NaYF4:Yb,Tm NCs. For deposition of small CdS NCs on these particles, we employ thioacetamide (TAA) as the sulfur source because it can slowly release S2− ions into the solution upon heating. When plenty of Cd2+ ions are adsorbed in the carbon shell, the nucleation of CdS NCs is initiated within or around the shell. Owing to the confinement of the polymer shell, fast growth of CdS nuclei into big NCs is restrained and small CdS NCs are thus produced in situ around the particle. These tiny CdS NCs have a tendency to aggregate together in order to reduce their surface energy. As a result, the formation of small nanoclusters is preferred under this system. At the same time, since the functional groups of the polymer shell can bind CdS nanoclusters through coordination, the produced CdS nanoclusters are covalently attached to the particle surface after deposition. However, it should be noted that CdS nanoclusters produced under these conditions have a relatively poor crystallinity (stirring at 80 °C for 1 h). To enhance their crystallinity, we employed microwave irradiation as a post-treatment procedure because this method is rapid, convenient and low cost. In particular, this method does not affect the morphology and structure of the samples since it happens in a very short time period and at relatively low temperature (80 °C for 10 min). After microwave irradiation, crystalline CdS nanoclusters can be obtained on the carbon shell-modified NaYF4:Yb,Tm NCs.TEM images of the samples at different preparation stages verify the above proposed synthetic strategy (see Fig. 1). Fig. 1A is a typical TEM image of the prepared NaYF4:Yb,Tm NCs. These NCs exhibit a hexagonal plate-like shape with a diameter of 200 nm (diagonal length) and a thickness of ca. 160 nm. These NCs are uniform in size and apt to self-assemble on the copper grid due to the use of oleic acid (OA) as a ligand during preparation. When these NaYF4:Yb,Tm NCs were modified with surfactant and hydrothermally treated in the glucose solution, obvious shells were produced on their surface, implying the formation of carbon shells on each NaYF4:Yb,Tm@C NC (see Fig. 1B). The carbon shell is around 30 nm in thickness. No free carbon sphere was produced as a byproduct in our case when the concentration of glucose was diluted to some extent (∼40 mg mL−1). After the formation of a carbon shell, these core–shell NPs can be well dispersed in water for chemical adsorption of Cd2+ ions in aqueous solution. The adsorption of metal ions can be verified by ICP measurement and this adsorption does not affect the morphology of the prepared NaYF4:Yb,Tm@C NPs (see Fig. 1C). When the Cd2+-saturated particles were mixed with TAA solution and treated under microwave irradiation, lots of CdS nanoclusters appeared on the NaYF4:Yb,Tm@C NPs, suggesting the success of the designed strategy. The formation of CdS nanoclusters instead of a compact CdS shell may provide some unique advantages. Firstly, this morphology allows more NIR light to reach the core NaYF4:Yb,Tm NCs due to the reduced scattering effect of the shell. Secondly, the reactant for catalysis can also be easily adsorbed by the carbon shell without any shielding effect from the shell. Sufficient adsorption of reactants is a crucial step for the whole photocatalytic process and it is also a key parameter to determine the overall efficiency of the photocatalysts.
 | ||
| Fig. 1 TEM images of samples obtained at different synthetic stages: (A) NaYF4:Yb,Tm NCs; (B) carbon-coated NaYF4:Yb,Tm NPs; (C) NaYF4:Yb,Tm@C after Cd2+ adsorption; (D) NaYF4:Yb,Tm@C@CdS NPs. | ||
3.2 SEM and TEM images
The morphologies of the prepared samples were characterized by both SEM and TEM (see Fig. 2). Fig. 2A displays a low-magnification SEM image of the NaYF4:Yb,Tm@C@CdS NPs. From this image, one can see that these particles are uniform in size and can be prepared on a large scale, showing the good productivity of this method. The average diameter of the particles is about 250 nm. At higher magnification, it is observed that these particles are big spheres to which many smaller particles are attached (see Fig. 2B). TEM images of these particles give a better insight into their structural information. As shown in Fig. 2C, one can observe the obvious core–shell structure of each particle according to the contrast between the different components in a single particle. The black cores are the NaYF4:Yb,Tm NCs with a diameter of 200 nm, and the gray shells of 30 nm constitute the crosslinked carbonous polymer. The black spots around the core–shell particles should be CdS nanoclusters. These small particles are around 10–30 nm in diameter. Under high-resolution TEM (see Fig. 2D), it is clearly observed that each CdS particle is not a single NC but consists of a cluster of tiny CdS NCs (also see Fig. S2†). One single cluster may contain 10–30 tiny NCs which are about 3–6 nm in size. Clear lattice fringes can be observed on each tiny NC in the clusters, showing the high crystallinity after microwave irradiation. The spacing between two fringes is 0.29 nm, matching well with the (200) plane of the cubic CdS crystal. The orientation of these nanoclusters is not along the same direction, suggesting that these tiny NCs randomly aggregate together, rather than following an oriented growth mechanism. The stability of the CdS nanoclusters on the carbon shell was also tested by repeating the ultrasonic treatment, washing and collection process. No obvious decrease in the number of CdS nanoclusters on each particle was found, suggesting that the carbon shell can firmly attach these nanoclusters due to the in situ deposition route and sufficient binding groups on the carbon shell.3.3 Phases and compositions of samples
Fig. 3A gives the XRD pattern of the NaYF4:Yb,Tm@C NPs before CdS coating. All the peaks are readily indexed to the pure hexagonal-phase NaYF4 crystal (JCPDS stand card no. 16-0334). No peak from cubic-phase NaYF4 crystals was found, showing the high purity of the doped NaYF4:Yb,Tm NCs. Note also that no peak from graphitized carbon was observed in the XRD patterns, suggesting that the shell is mainly composed of carbonous polymer rather than graphitized carbon species. This polymeric feature is important for the shell to sufficiently absorb Cd2+ ions around the NaYF4:Yb,Tm NCs. When the CdS nanoclusters form on the NaYF4:Yb,Tm@C NPs, two sets of diffraction peaks can be observed (see Fig. 3B). One set of peaks matches well with the hexagonal-phase NaYF4 crystals while the other set of peaks is consistent with cubic CdS crystals (standard JCPDS card no. 89-0440). Evidently, this result suggests that the purity and crystallinity of NaYF4:Yb,Tm cores were preserved during the post synthesis period and the newly created CdS nanoclusters were pure in phase. EDS analyses of the NaYF4:Yb,Tm@C@CdS NPs are shown in Fig. S3 and Table S1 (ESI†). The existence of elements such as Na, F, Y, Yb, C, S and Cd, confirms the composition of the NaYF4:Yb,Tm@C@CdS NPs. The signal from Cu and the high intensity signal from C in the EDS data result from the carbon-film coated copper grid. | ||
| Fig. 3 XRD patterns of the NaYF4:Yb,Tm@C NPs (A) and the NaYF4:Yb,Tm@C@CdS NPs (B). Standard XRD patterns of NaYF4 and CdS crystal are also given as references. | ||
Fig. 4 shows the high-resolution XPS spectra of the NaYF4:Yb,Tm@C@CdS NPs in which the elements Cd, S, Y, Na, Tm, and Yb are listed, respectively. The C 1s (284.1 eV) peak was used as an internal reference in the spectra. In Fig. 4A, the peaks of the binding energies at 405.1 eV and 411.7 eV are attributed to core levels of Cd3+ 3d5/2 and Cd3+ 3d3/2, respectively. Two peaks located at 161.6 eV and 163.6 eV in Fig. 4B can be assigned to the core levels of S2− 2p1/2 and S2− 2p3/2. These results are in agreement with the reported core levels of CdS crystals, implying that these nanoclusters are pure CdS NCs.32,33 Two XPS peaks at 161.2 eV and 159.3 eV are from Y3+ ions, corresponding to Y3+ 3d3/2 and Y3+ 3d5/2, respectively (see Fig. 4C). The element Na displays one characteristic peak at 1071.4 eV due to the core level of Na 1s (see Fig. 4D). In addition to the main elements in the NaYF4 NCs, the doping elements in these NCs can also be detected. The peaks at 171.9 eV, 174.2 eV (see Fig. 4E) and 182.3 eV (see Fig. 4F) are attributed to the Tm3+ ions and Yb3+ ions, respectively. The atomic ratios of these elements in the NaYF4:Yb,Tm@C@CdS NPs are listed in Table S2 (ESI†), and are close to the stoichiometric data of the sample.
 | ||
| Fig. 4 High-resolution XPS analyses of the NaYF4:Yb,Tm@C@CdS NPs: (A) Cd 3d, (B) S 2p, (C) Y 3d, (D) Na 1s, (E) Tm 4d and (F) Yb 4d. | ||
3.3 Upconversion emissions and UV-Vis absorption
NaYF4 NC is a typical nanoscale host-matrix for UC luminescence when doped with lanthanide ions.34,35 When Yb3+ and Tm3+ are co-doped inside, the NaYF4 NCs can produce strong UC emissions upon NIR excitation, in which Yb3+ serves as a sensitizer ion for continuous absorption of NIR light and Tm3+ functions as an activator ion to give various emissions. Fig. 5 displays the UC spectra of the NaYF4:Yb,Tm NCs under the excitation of a 980 nm wavelength. One can see that four UC peaks, namely two UV emissions and two blue emissions, can be observed in the spectra. The UV emissions centered at 349 nm and 362 nm are attributed to the 1I6 → 3F4 and 1D2 → 3H6 transitions of Tm3+ ions, while the two blue emissions located at 450 nm and 476 nm can be assigned to the 1D2 → 3F4 and 1G4 → 3H6 transitions of Tm3+ ions, respectively.29,36 After modification with a carbon shell, the UC emission of NaYF4:Yb,Tm NCs slightly decreased due to the light absorption and scattering effect of the carbon shell (see Fig. 6). When the CdS nanoclusters were deposited on the NaYF4:Yb,Tm@C NPs, strikingly, all four UC peaks dramatically reduced. Compared with the coated carbon shell, the CdS nanoclusters are less compact and do not have a strong scattering effect on the incident NIR light. Therefore, the remarkable decrease in UC emissions should be attributed to the absorption of the CdS nanoclusters. This result suggests that the UC emissions of core NaYF4:Yb,Tm NCs upon NIR excitation can be effectively absorbed by the CdS nanoclusters on the shell.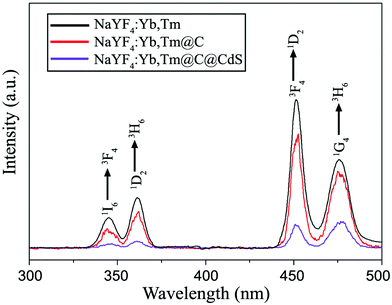 | ||
| Fig. 5 Upconversion PL spectra of the NaYF4:Yb,Tm NCs (black), NaYF4:Yb,Tm@C NPs (red) and NaYF4:Yb,Tm@C@CdS NPs (purple) under 980 nm excitation. | ||
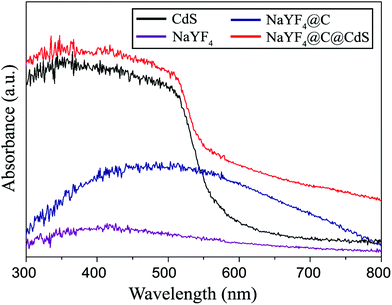 | ||
| Fig. 6 UV-Vis absorption spectra of the NaYF4:Yb,Tm NCs (purple), pure CdS NCs (black), NaYF4:Yb,Tm@C NPs (blue) and NaYF4:Yb,Tm@C@CdS NPs (red). | ||
UV-Vis absorption spectra of the NaYF4:Yb,Tm NCs before and after carbon modification are shown in Fig. 6. Before coating, the pure NaYF4:Yb,Tm NCs do not show obvious absorption in the Vis region. However, a low and wide absorption appears from the carbon-shell coated NaYF4:Yb,Tm NCs. This result is attributed to absorbance by crosslinked carbonous polymer in the shell because they are formed of many aromatic compounds. When CdS nanoclusters are deposited, the NaYF4:Yb,Tm@C@CdS NPs exhibit a strong absorption in the Vis band. This absorption is similar to the absorption spectrum of pure CdS NCs prepared under microwave irradiation without the addition of NaYF4:Yb,Tm@C seeds (see Fig. S4†). The bandgap of these pure CdS NCs is calculated to be 2.55 eV, showing the effect of quantum confinement compared to bulk CdS (2.4 eV).33 Comparing the UC spectra of NaYF4:Yb,Tm NCs with the absorption spectra of CdS nanoclusters, one can easily recognize that all four of the UC emissions of NaYF4:Yb,Tm NCs can be readily absorbed by the surface CdS NCs. This implies that a high utilization efficiency of the NIR light may be achieved through combining the NaYF4:Yb,Tm UCNs and CdS nanoclusters together.
3.4 Photocatalytic properties
Photocatalytic activity of the NaYF4:Yb,Tm@C@CdS NPs was assessed by the degradation of two model pollutants (RhB and MB). Through monitoring the characteristic absorption of RhB (553 nm) and MB (664 nm) at given intervals, the activity of the sample can be evaluated by a UV-Vis spectrometer. Fig. 7A shows the UV-Vis absorption spectra of the RhB solution with a small amount of NaYF4:Yb,Tm@C@CdS NPs (50 mg) under Vis irradiation (400–780 nm). The concentration of the RhB solution decreases very quickly under irradiation of the Vis light, showing the good catalytic activity of the sample. Considering that both the NaYF4:Yb,Tm NCs and the carbon shells have no catalytic effect under Vis light (see Fig. 7B), such Vis-driven activity should be ascribed to the CdS nanoclusters on the hybrid NPs. Interestingly, when the same photocatalytic experiments were carried out under the Vis-NIR band (400–2500 nm), a faster decrease in RhB concentration was observed (Fig. S5†). Plotting the relative concentration C/C0versus the degradation time, the difference in the activity of the sample under different irradiation bands can be observed (see Fig. 7B). Obviously, the NaYF4:Yb,Tm@C@CdS NPs show an enhanced photocatalytic activity under the Vis-NIR band compared to that under the pure Vis band. This result suggests that NaYF4:Yb,Tm NCs can improve the activity of CdS nanoclusters by utilizing the NIR light. To confirm this hypothesis, we also carried out the same experiment under a NIR laser and an obvious decrease in RhB concentration was also observed (see Fig. S6†).Although the carbon shell does not directly participate in the catalytic reaction, it has a strong adsorption capability for the dye molecules. In Fig. 7B, it was observed that the concentrations of the dyes notably decrease prior to light irradiation. Nearly fifteen percent of the dyes is absorbed by the catalysts regardless of the kind of irradiation band that will be applied. Considering that both NaYF4:Yb,Tm NCs and CdS nanoclusters only have slight adsorption capabilities for the dye molecules, such a high adsorption capacity should be ascribed to the carbon shells. This is an important feature for the catalysts to improve their total catalytic activity. We also synthesized pure CdS NCs without the NaYF4:Yb,Tm@C templates under microwave irradiation (see Fig. S4†). Under the Vis light, this sample indeed shows a low adsorption of dye molecules and lower activity than the NaYF4:Yb,Tm@C@CdS NPs (based on the same amount of CdS). This result further confirms that the carbon shell has a synergetic effect for the high activity of CdS nanoclusters.
The photocatalytic activity of the NaYF4:Yb,Tm@C@CdS NPs was also evaluated with other dyes such as MB molecules (see Fig. 7C and D). Enhanced activity under the Vis-NIR band and strong adsorption to dye molecules by the carbon shells can both be observed. These results confirm the indispensable roles of the NaYF4:Yb,Tm NCs and the carbon shells in the hybrid catalysts. The apparent rate constants of the degradation experiments were also employed to show the catalytic efficiency of the samples under different irradiation bands. As shown in Fig. S7 and Tables S3, S4,† the catalytic efficiency of NaYF4:Yb,Tm@C@CdS NPs under the Vis-NIR band is higher than under the Vis band, and also pure CdS NCs under the Vis band. These results further confirm the important function of UC cores and the carbon layer.
Photocatalytic activity of the mixture of NaYF4:Yb,Tm and CdS (similar amounts to those in NaYF4:Yb,Tm@C@CdS) was also assessed by the degradation of RhB solution (see Fig. S8†). The activity of this mixture is obviously lower than that of the NaYF4:Yb,Tm@C@CdS NPs under different irradiation bands, particularly under the NIR band, implying that the synthesis of uniform core–shell nanostructures with a carbon layer is crucial to their good photocatalytic properties. On the other hand, we have also attempted the synthesis of NaYF4:Yb,Tm@C NPs with different carbon thicknesses (see Fig. S9†). The results suggested that a thicker carbon shell would reduce the UC emission of samples due to the absorption and light scattering effect. But a fairly thin shell would lower the adsorption to dye molecules for subsequent catalytic applications (see Fig. S10†). As such, a moderate carbon shell (i.e., 30 nm) between the NaYF4:Yb,Tm and CdS nanoclusters is thus preferred for the consideration of both energy transfer and dye adsorption.
3.5 Working mechanism of the hybrid photocatalysts
It is well established that photocatalytic oxidation of organic pollutants is initiated by reactive species such as photogenerated holes (h+), hydroxyl radicals (˙OH) and superoxide radicals (˙O2−). In our experiments, the active species generated from the NaYF4:Yb,Tm@C@CdS NPs were also investigated under NIR light. Three kinds of scavengers (i.e., ammonium oxalate (AO), tertiary butanol (t-BuOH) and benzoquinone (BQ)), were separately introduced to the degradation process of RhB molecules for trapping the h+, ˙OH and ˙O2−, respectively. As shown in Fig. 8, the degradation rate is significantly restrained in the presence of BQ (˙O2− scavenger), implying that ˙O2− should be a major contributor to the decomposition of the dye molecules. When AO was introduced to the system to suppress the photogenerated h+, the degradation rate was also greatly reduced, revealing that h+ is also a main reactive species in the photocatalytic oxidation process. However, if t-BuOH was added as a scavenger of ˙OH, the degradation rate only showed a slight decrease, suggesting that ˙OH is not a dominant active species. This result may be attributed to the fact that the valence band (VB) edge of CdS (∼1.65 V vs. NHE, pH = 7) is less positive than the standard potential of ˙OH/OH− (∼2.38 V vs. NHE, pH = 7).37,38 As such, the photogenerated h+ cannot efficiently oxidize OH− groups to ˙OH radicals. The existence of very few ˙OH radicals in the system suggests they may be evolved from ˙O2− radicals. We also conducted the above three scavenger experiments under Vis light and found that the results were very similar to those under the NIR light. This result confirms that CdS nanoclusters are the catalytic part for generating reactive species in the hybrid photocatalysts and the NaYF4:Yb,Tm NPs serve as light transducers. | ||
| Fig. 8 Photocatalytic activity of the NaYF4:Yb,Tm @C@CdS NPs in the presence of different scavengers under NIR light for degradation of the RhB solution. | ||
Based on the UC spectra and the detected reactive species, the enhanced mechanism of the hybrid NaYF4:Yb,Tm@C@CdS photocatalysts under NIR light is illustrated in Scheme 2. Firstly, the Yb3+ ions serve as sensitizer ions to absorb the NIR light in the Yb3+ and Tm3+ co-doped NaYF4 NCs. When the excited Yb3+ ions relax from the 2F5/2 level to the 2F7/2 level, they will successively transfer energy to the nearby Tm3+ ions. Through a typical energy transfer upconversion (ETU) process, the Tm3+ ions are excited to the 3H5 level and then relax nonradiatively to the 3F4 level. Successive ET from Yb3+ to Tm3+ leads to the population of the 3F2 level of Tm3+.21 Afterwards, the 3F2 level relaxes to the 3H4 level and then is excited to the 1G4 level by ET from other excited Yb3+ ions.39 Because of the large energy mismatch (about 3500 cm−1), the 1G4 level cannot be directly populated from the 1D2 level by the fourth photon from Yb3+via an ET process. The population of the 1D2 level may be realized by two cross relaxation processes: 3F2 + 3H4 → 3H6 + 1D2 and 1G4 + 3H4 → 3F4 + 1D2.40 After that, the excited Tm3+ ions fall to lower energy levels: 1I6 → 3F4, 1D2 → 3F4, 1G4 → 3H6, 1D2 → 3H6, and 1G4 → 3F4, leading to emission at 345 nm, 368 nm, 450 nm, and 475 nm, respectively.39,40 The energy gap of CdS is about 2.4 eV, and hence all four UC emissions from the NaYF4:Yb,Tm NCs can be absorbed by the CdS nanoclusters. Under the excitation of these UC emissions, the activated CdS nanoclusters produce photoelectrons (e−) and holes (h+) in the CB and VB bands, respectively, which then migrate from the inner region to the surface. Upon reaching the dye molecules, the h+ can directly function as oxidants while the e− react with O2 and produce ˙O2− species. Both the h+ and ˙O2− can be directly utilized for the photocatalytic oxidation of dye molecules. Though the carbon layer does not directly participate in the reaction, it also plays two important roles: (1) serving as an adsorber to enrich the dye molecules around the photocatalysts; and (2) working as a firm substrate to fix the CdS nanocluster around the carbon-coated NaYF4:Yb,Tm NCs.
 | ||
| Scheme 2 Diagrams of the energy levels for the upconversion process and the NIR light-driven photocatalytic mechanism of the NaYF4:Yb,Tm@C@CdS NPs. | ||
4. Conclusions
In summary, we have developed a facile strategy to construct hybrid NIR-activated photocatalysts with a uniform core–shell structure. High-quality hydrophobic NaYF4:Yb,Tm NCs were modified with a carbon layer through the reverse micelle method. The carbon layer-modified NaYF4:Yb,Tm NCs not only become hydrophilic but also provide a good substrate for CdS deposition. The developed NaYF4:Yb,Tm@C@CdS NPs can work under NIR light as photocatalysts through upconverting the NIR photons to be UV and Vis ones. Due to the NIR utilization and the adsorption capability of the carbon layer, the developed photocatalysts exhibit good photocatalytic activity under Vis light and obviously enhanced performance under the Vis-NIR light. The working mechanism of this hybrid NIR-activated photocatalyst has also been proposed.Acknowledgements
The authors acknowledge financial support from the National Nature Science Foundation of China (no. 21273203) and Zhejiang Provincial Natural Science Foundation (no. LR15B010001, LQ16B010001 and LR12B040001).References
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- C. C. Chen, W. H. Ma and J. C. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- S. Bai, J. Jiang, Q. Zhang and Y. J. Xiong, Chem. Soc. Rev., 2015, 44, 2893–2939 RSC.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- P. Wang, J. Wang, T. S. Ming, X. F. Wang, H. G. Yu, J. G. Yu, Y. G. Wang and M. Lei, ACS Appl. Mater. Interfaces, 2013, 5, 2924–2929 CAS.
- L. Li, S. Q. Zhou, E. J. Chen, R. Qiao, Y. J. Zhong, Y. Zhang and Z. Q. Li, J. Mater. Chem. A, 2015, 3, 2234–2241 CAS.
- W. J. Zhou, Z. Y. Yin, Y. P. Du, X. Huang, Z. Y. Zeng, Z. X. Fan, H. Liu, J. Y. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- W. Zhao, W. H. Ma, C. C. Chen, J. C. Zhao and Z. G. Shuai, J. Am. Chem. Soc., 2004, 126, 4782–4783 CrossRef CAS PubMed.
- M. Liu, X. Q. Qiu, M. Miyauchi and K. Hashimoto, J. Am. Chem. Soc., 2013, 135, 10064–10072 CrossRef CAS PubMed.
- A. Kubacka, M. F. García and G. Colón, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- Y. T. Dong, J. Choi, H. K. Jeong and D. H. Son, J. Am. Chem. Soc., 2015, 137, 5549–5554 CrossRef CAS PubMed.
- S. Q. Huang, N. W. Zhu, Z. Y. Lou, L. Gu, C. Miao, H. P. Yuan and A. D. Shan, Nanoscale, 2014, 6, 1362–1368 RSC.
- W. Wang, M. Y. Ding, C. H. Lu, Y. R. Ni and Z. Z. Xu, Appl. Catal., B, 2014, 144, 379–385 CrossRef CAS.
- Y. W. Zhang and Z. L. Hong, Nanoscale, 2013, 5, 8930–8933 RSC.
- W. K. Su, M. M. Zheng, L. Li, K. Wang, R. Qiao, Y. J. Zhong, Y. Hu and Z. Q. Li, J. Mater. Chem. A, 2014, 2, 13486–13491 CAS.
- Y. X. Guo, H. W. Huang, Y. He, N. Tian, T. R. Zhang, P. K. Chu, Q. An and Y. H. Zhang, Nanoscale, 2015, 7, 11702–11711 RSC.
- C. H. Li, F. Wang, J. Zhu and J. C. Yu, Appl. Catal., B, 2010, 100, 433–439 CrossRef CAS.
- G. Y. Chen, H. L. Qiu, P. N. Prasad and X. Y. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, Acc. Chem. Res., 2014, 47, 1378–1385 CrossRef CAS PubMed.
- Y. Hu, Y. Liu, H. S. Qian, Z. Q. Li and J. F. Chen, Langmuir, 2010, 26, 18570–18575 CrossRef CAS PubMed.
- D. A. Bulushev, L. G. Bulusheva, S. Beloshapkin, T. O'Connor, A. V. Okotrub and K. M. Ryan, ACS Appl. Mater. Interfaces, 2015, 7, 8719–8726 CAS.
- M. J. Zhou, Y. Hu, Y. Liu, W. L. Yang and H. S. Qian, CrystEngComm, 2012, 14, 7686–7693 RSC.
- A. J. Romero-Anaya, M. Ouzzine, M. A. Lillo-Ródenas and A. Linares-Solano, Carbon, 2014, 68, 296–307 CrossRef CAS.
- Y. Hu, X. H. Gao, L. Yu, Y. R. Wang, J. Q. Ning, S. J. Xu and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 5636–5639 CrossRef CAS PubMed.
- P. Zabek, J. Eberl and H. Kisch, Photochem. Photobiol. Sci., 2009, 8, 264–269 CAS.
- S. Sato, Langmuir, 1988, 4, 1156–1161 CrossRef CAS.
- Z. Q. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765–4769 CrossRef CAS.
- Y. Zhao, W. Li, X. Zhao, D. P. Wang and S. X. Liu, Mater. Res. Innovations, 2013, 17, 546–551 CrossRef.
- J. J. Li, Y. A. Wang, W. Z. Guo, J. C. Keay, T. D. Mishima, M. B. Johnson and X. G. Peng, J. Am. Chem. Soc., 2003, 125, 12567–12575 CrossRef CAS PubMed.
- J. G. Yu, Y. F. Yu, P. Zhou, W. Xiao and B. Cheng, Appl. Catal., B, 2014, 156–157, 184–191 CrossRef CAS.
- G. F. Wang, Q. Peng and Y. D. Li, Acc. Chem. Res., 2011, 44, 322–332 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- C. X. Li, Z. W. Quan, J. Yang, P. P. Yang and J. Lin, Inorg. Chem., 2007, 46, 6329–6337 CrossRef CAS PubMed.
- T. Simon, N. Bouchonville, M. J. Berr, A. Vaneski, A. Adrovic, D. Volbers, R. Wyrwich, M. Doblinger, A. S. Susha, A. L. Roach, F. Jackel, J. K. Stolarczyk and J. Feldmann, Nat. Mater., 2014, 13, 1013–1018 CrossRef CAS PubMed.
- S. Pasternak and Y. Paz, ChemPhysChem, 2013, 14, 2059–2070 CrossRef CAS PubMed.
- J. Zhou, Z. Liu and F. Y. Li, Chem. Soc. Rev., 2012, 41, 1323–1349 RSC.
- Y. N. Tang, W. H. Di, X. S. Zhai, R. Y. Yang and W. P. Qin, ACS Catal., 2013, 3, 405–412 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr06806a |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2016 |



